Continuous, but not intermittent, regimens of hypoxia prevent and reverse ataxia in a murine model of Friedreich's ataxia
- PMID: 37260376
- PMCID: PMC10407700
- DOI: 10.1093/hmg/ddad091
Continuous, but not intermittent, regimens of hypoxia prevent and reverse ataxia in a murine model of Friedreich's ataxia
Abstract
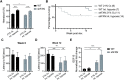
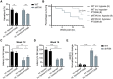
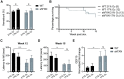
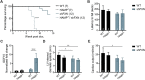
Friedreich's ataxia (FA) is a devastating, multi-systemic neurodegenerative disease affecting thousands of people worldwide. We previously reported that oxygen is a key environmental variable that can modify FA pathogenesis. In particular, we showed that chronic, continuous normobaric hypoxia (11% FIO2) prevents ataxia and neurological disease in a murine model of FA, although it did not improve cardiovascular pathology or lifespan. Here, we report the pre-clinical evaluation of seven 'hypoxia-inspired' regimens in the shFxn mouse model of FA, with the long-term goal of designing a safe, practical and effective regimen for clinical translation. We report three chief results. First, a daily, intermittent hypoxia regimen (16 h 11% O2/8 h 21% O2) conferred no benefit and was in fact harmful, resulting in elevated cardiac stress and accelerated mortality. The detrimental effect of this regimen is likely owing to transient tissue hyperoxia that results when daily exposure to 21% O2 combines with chronic polycythemia, as we could blunt this toxicity by pharmacologically inhibiting polycythemia. Second, we report that more mild regimens of chronic hypoxia (17% O2) confer a modest benefit by delaying the onset of ataxia. Third, excitingly, we show that initiating chronic, continuous 11% O2 breathing once advanced neurological disease has already started can rapidly reverse ataxia. Our studies showcase both the promise and limitations of candidate hypoxia-inspired regimens for FA and underscore the need for additional pre-clinical optimization before future translation into humans.
© The Author(s) 2023. Published by Oxford University Press.
Figures


References
-
- Pandolfo, M. (2012) Friedreich ataxia. Handb. Clin. Neurol., 103, 275–294. - PubMed
-
- Harding, A.E. (1981) Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain, 104, 589–620. - PubMed
-
- Tsou, A.Y., Paulsen, E.K., Lagedrost, S.J., Perlman, S.L., Mathews, K.D., Wilmot, G.R., Ravina, B., Koeppen, A.H. and Lynch, D.R. (2011) Mortality in Friedreich ataxia. J. Neurol. Sci., 307, 46–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

