Structure, Assembly, and Function of Flagella Responsible for Bacterial Locomotion
- PMID: 37260402
- PMCID: PMC10729930
- DOI: 10.1128/ecosalplus.esp-0011-2023
Structure, Assembly, and Function of Flagella Responsible for Bacterial Locomotion
Abstract
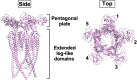
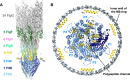
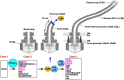
Many motile bacteria use flagella for locomotion under a variety of environmental conditions. Because bacterial flagella are under the control of sensory signal transduction pathways, each cell is able to autonomously control its flagellum-driven locomotion and move to an environment favorable for survival. The flagellum of Salmonella enterica serovar Typhimurium is a supramolecular assembly consisting of at least three distinct functional parts: a basal body that acts as a bidirectional rotary motor together with multiple force generators, each of which serves as a transmembrane proton channel to couple the proton flow through the channel with torque generation; a filament that functions as a helical propeller that produces propulsion; and a hook that works as a universal joint that transmits the torque produced by the rotary motor to the helical propeller. At the base of the flagellum is a type III secretion system that transports flagellar structural subunits from the cytoplasm to the distal end of the growing flagellar structure, where assembly takes place. In recent years, high-resolution cryo-electron microscopy (cryoEM) image analysis has revealed the overall structure of the flagellum, and this structural information has made it possible to discuss flagellar assembly and function at the atomic level. In this article, we describe what is known about the structure, assembly, and function of Salmonella flagella.
Keywords: bacterial flagellum; chemotaxis; cryoEM image analysis; energy coupling; flagellar assembly; flagellar gene regulation; motility; torque generation; transmembrane proton channel; type III secretion system.
Figures










Similar articles
-
Architecture and Assembly of the Bacterial Flagellar Motor Complex.Subcell Biochem. 2021;96:297-321. doi: 10.1007/978-3-030-58971-4_8. Subcell Biochem. 2021. PMID: 33252734 Review.
-
Structure and Dynamics of the Bacterial Flagellar Motor Complex.Biomolecules. 2024 Nov 22;14(12):1488. doi: 10.3390/biom14121488. Biomolecules. 2024. PMID: 39766194 Free PMC article. Review.
-
Structure and function of the bi-directional bacterial flagellar motor.Biomolecules. 2014 Feb 18;4(1):217-34. doi: 10.3390/biom4010217. Biomolecules. 2014. PMID: 24970213 Free PMC article. Review.
-
In Situ Structures of Polar and Lateral Flagella Revealed by Cryo-Electron Tomography.J Bacteriol. 2019 Jun 10;201(13):e00117-19. doi: 10.1128/JB.00117-19. Print 2019 Jul 1. J Bacteriol. 2019. PMID: 31010901 Free PMC article.
-
Flagella-Driven Motility of Bacteria.Biomolecules. 2019 Jul 14;9(7):279. doi: 10.3390/biom9070279. Biomolecules. 2019. PMID: 31337100 Free PMC article. Review.
Cited by
-
Unraveling the dynamics of Xanthomonas' flagella: insights into host-pathogen interactions.PeerJ. 2024 Oct 21;12:e18204. doi: 10.7717/peerj.18204. eCollection 2024. PeerJ. 2024. PMID: 39465145 Free PMC article. Review.
-
Rotation of the Fla2 flagella of Cereibacter sphaeroides requires the periplasmic proteins MotK and MotE that interact with the flagellar stator protein MotB2.PLoS One. 2024 Mar 20;19(3):e0298028. doi: 10.1371/journal.pone.0298028. eCollection 2024. PLoS One. 2024. PMID: 38507361 Free PMC article.
-
Mapping the loss of flagellar motility across the tree of life.ISME J. 2025 Jan 2;19(1):wraf111. doi: 10.1093/ismejo/wraf111. ISME J. 2025. PMID: 40509753 Free PMC article.
-
Resistance Response and Regulatory Mechanisms of Ciprofloxacin-Induced Resistant Salmonella Typhimurium Based on Comprehensive Transcriptomic and Metabolomic Analysis.Antibiotics (Basel). 2025 Jul 29;14(8):767. doi: 10.3390/antibiotics14080767. Antibiotics (Basel). 2025. PMID: 40867962 Free PMC article.
-
A new target of multiple lysine methylation in bacteria.J Bacteriol. 2025 Jan 31;207(1):e0032524. doi: 10.1128/jb.00325-24. Epub 2024 Dec 11. J Bacteriol. 2025. PMID: 39660925 Free PMC article.
References
-
- Macnab RM. 1996. Flagella and motility, p 123–145. In Neidhardt FC, Curtiss R, III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

