Update on Adipose Tissue and Cancer
- PMID: 37260403
- PMCID: PMC10638602
- DOI: 10.1210/endrev/bnad015
Update on Adipose Tissue and Cancer
Abstract
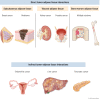
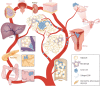
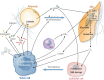
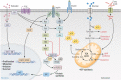
Adipose tissue is the largest endocrine organ and an accepted contributor to overall energy homeostasis. There is strong evidence linking increased adiposity to the development of 13 types of cancer. With increased adiposity comes metabolic dysfunction and insulin resistance, and increased systemic insulin and glucose support the growth of many cancers, including those of the colon and endometrium. There is also an important direct crosstalk between adipose tissue and various organs. For instance, the healthy development and function of the mammary gland, as well as the development, growth, and progression of breast cancer, are heavily impacted by the breast adipose tissue in which breast epithelial cells are embedded. Cells of the adipose tissue are responsive to external stimuli, including overfeeding, leading to remodeling and important changes in the secretion of factors known to drive the development and growth of cancers. Loss of factors like adiponectin and increased production of leptin, endotrophin, steroid hormones, and inflammatory mediators have been determined to be important mediators of the obesity-cancer link. Obesity is also associated with a structural remodeling of the adipose tissue, including increased localized fibrosis and disrupted angiogenesis that contribute to the development and progression of cancers. Furthermore, tumor cells feed off the adipose tissue, where increased lipolysis within adipocytes leads to the release of fatty acids and stromal cell aerobic glycolysis leading to the increased production of lactate. Both have been hypothesized to support the higher energetic demands of cancer cells. Here, we aim to provide an update on the state of the literature revolving around the role of the adipose tissue in cancer initiation and progression.
Keywords: adipokines; adipose tissue; cancer; obesity.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- World Health Organization . World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals. World Health Organization; 2022.
-
- He Q, Xia B, Liu A, et al. . Association of body composition with risk of overall and site-specific cancers: a population-based prospective cohort study. Int J Cancer. 2021;149(7):1435‐1447. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

