Aflibercept for long-term treatment of diabetic macular edema and proliferative diabetic retinopathy: a meta-analysis
- PMID: 37260449
- PMCID: PMC10227619
- DOI: 10.3389/fendo.2023.1144422
Aflibercept for long-term treatment of diabetic macular edema and proliferative diabetic retinopathy: a meta-analysis
Abstract
Purpose: This meta-analysis compared the long-term (12 months or 24 months) efficacy and safety of intravitreal aflibercept injection (IAI) for diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR).
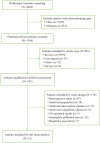
Methods: We selected 16 randomized controlled trials (RCTs) performed after 2015 that had a minimum of 12 months and up to 24 months of treatment and conducted a meta-analysis with Review Manager version 5.3. Visual acuity (VA), central subfield thickness (CST) and adverse events were the outcomes selected for evaluation from the eligible studies.
Results: Based on 16 RCTs, we evaluated a total of 7125 patients. For PDR and severe DME with poor baseline vision, after a minimum of 12 months and up to 24 months of treatment, the aflibercept treatment group obtained better VA improvement than the focal/grid laser photocoagulation treatment group (MD=13.30; 95%CI: 13.01~13.58; P<0.001) or other treatments (ranibizumab, focal/grid laser photocoagulation, PRP, et al.) group (MD=1.10; 95%CI: 1.05~1.16; P<0.001). In addition, the aflibercept treatment group got higher CST reduction than the focal/grid laser photocoagulation treatment (MD=-33.76; 95%CI: -45.53 ~ -21.99; P<0.001) or other treatments (ranibizumab, focal/grid laser photocoagulation, et al.) group (MD=-33.76; 95%CI: -45.53 ~ -21.99; P<0.001). There was no significant difference in the overall incidence of ocular and non-ocular adverse events in each treatment group.
Conclusions: This meta-analysis showed that the advantages of IAI are obvious in the management of DME and PDR with poor baseline vision for long-term observation (a minimum of 12 months and up to 24 months) with both VA improvement and CST reduction. Applied IAI separately trended to be more effective than panretinal photocoagulation separately in VA improvement for PDR. More parameters should be required to assess functional and anatomic outcomes.
Keywords: aflibercept; anti-vascular endothelial growth factor; diabetic macular edema; focal/grid laser photocoagulation; meta-analysis; panretinal photocoagulation; proliferative diabetic retinopathy.
Copyright © 2023 Xie, Lian, Zhang, Feng, Wang, Yuan, Shi and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer MC declared a shared affiliation with the authors to the handling editor at the time of review.
Figures






Similar articles
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2018 Oct 16;10(10):CD007419. doi: 10.1002/14651858.CD007419.pub6. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Jun 27;2023(6):CD007419. doi: 10.1002/14651858.CD007419.pub7. PMID: 30325017 Free PMC article. Updated.
-
Panretinal Photocoagulation Versus Ranibizumab for Proliferative Diabetic Retinopathy: Factors Associated with Vision and Edema Outcomes.Ophthalmology. 2018 Nov;125(11):1776-1783. doi: 10.1016/j.ophtha.2018.04.039. Epub 2018 Jul 3. Ophthalmology. 2018. PMID: 29980333 Free PMC article. Clinical Trial.
-
Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion.Cochrane Database Syst Rev. 2020 Jul 7;7(7):CD009510. doi: 10.1002/14651858.CD009510.pub3. Cochrane Database Syst Rev. 2020. PMID: 32633861 Free PMC article.
-
Recent clinically relevant highlights from the Diabetic Retinopathy Clinical Research Network.Curr Opin Ophthalmol. 2018 May;29(3):199-205. doi: 10.1097/ICU.0000000000000472. Curr Opin Ophthalmol. 2018. PMID: 29528861 Review.
-
Aflibercept 5+PRN with retinal laser photocoagulation is more effective than retinal laser photocoagulation alone and aflibercept 3+PRN with retinal laser photocoagulation in patients with high-risk proliferative diabetic retinopathy and diabetic macular edema: a 12-month clinical trial.Front Endocrinol (Lausanne). 2024 Feb 22;15:1286736. doi: 10.3389/fendo.2024.1286736. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38455651 Free PMC article.
Cited by
-
Intravitreal therapy for the management of diabetic retinopathy: A concise review.World J Exp Med. 2024 Dec 20;14(4):99235. doi: 10.5493/wjem.v14.i4.99235. eCollection 2024 Dec 20. World J Exp Med. 2024. PMID: 39713073 Free PMC article. Review.
-
Proliferative Diabetic Retinopathy in Nigerians: Treatment Methods, Visual Outcomes, and Predictors of Poor Outcomes.J West Afr Coll Surg. 2025 Jul-Sep;15(3):335-343. doi: 10.4103/jwas.jwas_76_24. Epub 2025 Feb 4. J West Afr Coll Surg. 2025. PMID: 40586066 Free PMC article.
References
-
- Yonekawa Y, Modi YS, Kim LA, Skondra D, Kim JE, Wykoff CC. American society of retina specialists clinical practice guidelines on the management of nonproliferative and proliferative diabetic retinopathy without diabetic macular edema. J Vitreoretin Dis (2020) 4(2):125–35. doi: 10.1177/2474126419893829 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

