Enhanced detoxification of Cr6+ by Shewanella oneidensis via adsorption on spherical and flower-like manganese ferrite nanostructures
- PMID: 37260478
- PMCID: PMC10228370
- DOI: 10.1039/d2na00691j
Enhanced detoxification of Cr6+ by Shewanella oneidensis via adsorption on spherical and flower-like manganese ferrite nanostructures
Abstract
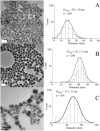
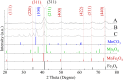
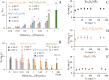
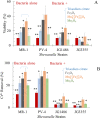
Maximizing the safe removal of hexavalent chromium (Cr6+) from waste streams is an increasing demand due to the environmental, economic and health benefits. The integrated adsorption and bio-reduction method can be applied for the elimination of the highly toxic Cr6+ and its detoxification. This work describes a synthetic method for achieving the best chemical composition of spherical and flower-like manganese ferrite (MnxFe3-xO4) nanostructures (NS) for Cr6+ adsorption. We selected NS with the highest adsorption performance to study its efficiency in the extracellular reduction of Cr6+ into a trivalent state (Cr3+) by Shewanella oneidensis (S. oneidensis) MR-1. MnxFe3-xO4 NS were prepared by a polyol solvothermal synthesis process. They were characterised by powder X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectrometry (XPS), dynamic light scattering (DLS) and Fourier transform-infrared (FTIR) spectroscopy. The elemental composition of MnxFe3-xO4 was evaluated by inductively coupled plasma atomic emission spectroscopy. Our results reveal that the oxidation state of the manganese precursor significantly affects the Cr6+ adsorption efficiency of MnxFe3-xO4 NS. The best adsorption capacity for Cr6+ is 16.8 ± 1.6 mg Cr6+/g by the spherical Mn0.22+Fe2.83+O4 nanoparticles at pH 7, which is 1.4 times higher than that of Mn0.8Fe2.2O4 nanoflowers. This was attributed to the relative excess of divalent manganese in Mn0.22+Fe2.83+O4 based on our XPS analysis. The lethal concentration of Cr6+ for S. oneidensis MR-1 was 60 mg L-1 (determined by flow cytometry). The addition of Mn0.22+Fe2.83+O4 nanoparticles to S. oneidensis MR-1 enhanced the bio-reduction of Cr6+ 2.66 times compared to the presence of the bacteria alone. This work provides a cost-effective method for the removal of Cr6+ with a minimum amount of sludge production.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Novel green strategy for CuO-ZnO-C nanocomposites fabrication using marigold (Tagetes spp.) flower petals extract with and without CTAB treatment for adsorption of Cr(VI) and Congo red dye.J Environ Manage. 2021 Jul 15;290:112615. doi: 10.1016/j.jenvman.2021.112615. Epub 2021 Apr 24. J Environ Manage. 2021. PMID: 33906117
-
Correction: Enhanced detoxification of Cr6+ by Shewanella oneidensis via adsorption on spherical and flower-like manganese ferrite nanostructures.Nanoscale Adv. 2023 May 16;5(11):3114. doi: 10.1039/d3na90043f. eCollection 2023 May 30. Nanoscale Adv. 2023. PMID: 37260490 Free PMC article.
-
Composition-Tunable Ultrasmall Manganese Ferrite Nanoparticles: Insights into their In Vivo T1 Contrast Efficacy.Theranostics. 2019 Feb 28;9(6):1764-1776. doi: 10.7150/thno.31233. eCollection 2019. Theranostics. 2019. PMID: 31037137 Free PMC article.
-
Enhancement of hexavalent chromium reduction by Shewanella oneidensis MR-1 in presence of copper nanoparticles via stimulating bacterial extracellular electron transfer and environmental adaptability.Bioresour Technol. 2022 Oct;361:127686. doi: 10.1016/j.biortech.2022.127686. Epub 2022 Jul 25. Bioresour Technol. 2022. PMID: 35901865
-
Iron mineral-humic acid complex enhanced Cr(VI) reduction by Shewanella oneidensis MR-1.Chemosphere. 2020 May;247:125902. doi: 10.1016/j.chemosphere.2020.125902. Epub 2020 Jan 11. Chemosphere. 2020. PMID: 31978657
Cited by
-
Cu/Cu2O/NH2-MIL-88B (Fe) heterojunction as the photocatalyst to remove hexavalent chromium heavy metal ions in water.RSC Adv. 2025 Jan 24;15(4):2462-2469. doi: 10.1039/d4ra08143a. eCollection 2025 Jan 23. RSC Adv. 2025. PMID: 39867318 Free PMC article.
References
-
- Wang B. Liu B. Gu J. Somers M. A. J. Surf. Coat. Technol. 2022;438:128408–128422. doi: 10.1016/j.surfcoat.2022.128408. - DOI
-
- Tang H. Peng Z. Gu F. Yang L. Tian W. Zhong Q. Rao M. Li G. Jiang T. Ceram. Int. 2021;47:10809–10818. doi: 10.1016/j.ceramint.2020.12.198. - DOI
-
- Xing J. Pailthorpe M. T. J. Soc. Dyers Colour. 2000;116:91–93.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

