Stille vs. Suzuki - cross-coupling for the functionalization of diazocines
- PMID: 37260568
- PMCID: PMC10227463
- DOI: 10.1039/d3ra02988c
Stille vs. Suzuki - cross-coupling for the functionalization of diazocines
Abstract
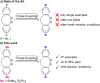
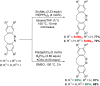
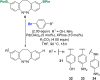
Diazocines are azobenzene derived macrocyclic photoswitches with well resolved photostationary states for the (E)- and (Z)-isomers, which improves their addressability by light. In this work, effective procedures for the stannylation and borylation of diazocines in different positions are reported. Their use in Stille cross-coupling and Suzuki cross-coupling reactions with organic bromides is demonstrated in yields of 47-94% (Stille cross-coupling) and 0-95% (Suzuki cross-coupling), respectively.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

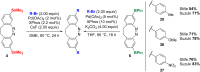
References
-
- Siewertsen R. Neumann H. Buchheim-Stehn B. Herges R. Näther C. Renth F. Temps F. J. Am. Chem. Soc. 2009;131:15594–15595. - PubMed
- Deo C. Bogliotti N. Métivier R. Retailleau P. Xie J. Chem.–Eur. J. 2016;22:9092–9096. - PubMed
- Hammerich M. Schutt C. Stahler C. Lentes P. Rohricht F. Hoppner R. Herges R. J. Am. Chem. Soc. 2016;138:13111–13114. - PubMed
-
- Burk M. H. Schröder S. Moormann W. Langbehn D. Strunskus T. Rehders S. Herges R. Faupel F. Macromolecules. 2020;53:1164–1170.
- Burk M. H. Langbehn D. Hernández Rodríguez G. Reichstein W. Drewes J. Schröder S. Rehders S. Strunskus T. Herges R. Faupel F. ACS Appl. Polym. Mater. 2021;3:1445–1456.
- Li S. Colaco R. Staubitz A. ACS Appl. Polym. Mater. 2022;4:6825–6833.
-
- Li S. Han G. Zhang W. Macromolecules. 2018;51:4290–4297.
-
- Cabré G. Garrido-Charles A. González-Lafont À. Moormann W. Langbehn D. Egea D. Lluch J. M. Herges R. Alibés R. Busqué F. Gorostiza P. Hernando J. Org. Lett. 2019;21:3780–3784. - PubMed
- Ewert J. Heintze L. Jorda-Redondo M. von Glasenapp J. S. Nonell S. Bucher G. Peifer C. Herges R. J. Am. Chem. Soc. 2022;144:15059–15071. - PubMed
- Reynders M. Matsuura B. S. Bérouti M. Simoneschi D. Marzio A. Pagano M. Trauner D. Sci. Adv. 2020;6:eaay5064. - PMC - PubMed
- Samanta S. Qin C. Lough A. J. Woolley G. A. Angew. Chem., Int. Ed. 2012;51:6452–6455. - PubMed
LinkOut - more resources
Full Text Sources

