Adjuvant capecitabine in triple negative breast cancer patients with residual disease after neoadjuvant treatment: real-world evidence from CaRe, a multicentric, observational study
- PMID: 37260975
- PMCID: PMC10227592
- DOI: 10.3389/fonc.2023.1152123
Adjuvant capecitabine in triple negative breast cancer patients with residual disease after neoadjuvant treatment: real-world evidence from CaRe, a multicentric, observational study
Abstract
Background: In triple negative breast cancer patients treated with neoadjuvant chemotherapy, residual disease at surgery is the most relevant unfavorable prognostic factor. Current guidelines consider the use of adjuvant capecitabine, based on the results of the randomized CREATE-X study, carried out in Asian patients and including a small subset of triple negative tumors. Thus far, evidence on Caucasian patients is limited, and no real-world data are available.
Methods: We carried out a multicenter, observational study, involving 44 oncologic centres. Triple negative breast cancer patients with residual disease, treated with adjuvant capecitabine from January 2017 through June 2021, were recruited. We primarily focused on treatment tolerability, with toxicity being reported as potential cause of treatment discontinuation. Secondarily, we assessed effectiveness in the overall study population and in a subset having a minimum follow-up of 2 years.
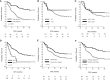
Results: Overall, 270 patients were retrospectively identified. The 50.4% of the patients had residual node positive disease, 7.8% and 81.9% had large or G3 residual tumor, respectively, and 80.4% a Ki-67 >20%. Toxicity-related treatment discontinuation was observed only in 10.4% of the patients. In the whole population, at a median follow-up of 15 months, 2-year disease-free survival was 62%, 2 and 3-year overall survival 84.0% and 76.2%, respectively. In 129 patients with a median follow-up of 25 months, 2-year disease-free survival was 43.4%, 2 and 3-year overall survival 78.0% and 70.8%, respectively. Six or more cycles of capecitabine were associated with more favourable outcomes compared with less than six cycles.
Conclusion: The CaRe study shows an unexpectedly good tolerance of adjuvant capecitabine in a real-world setting, although effectiveness appears to be lower than that observed in the CREATE-X study. Methodological differences between the two studies impose significant limits to comparability concerning effectiveness, and strongly invite further research.
Keywords: adjuvant capecitabine; neoadjuvant treatment; residual tumors; treatment discontinuation; triple negative breast cancer.
Copyright © 2023 Di Lisa, Krasniqi, Pizzuti, Barba, Cannita, De Giorgi, Borella, Foglietta, Cariello, Ferro, Picardo, Mitidieri, Sini, Stani, Tonini, Santini, La Verde, Gambaro, Grassadonia, Tinari, Garrone, Sarobba, Livi, Meattini, D’Auria, Vergati, Gamucci, Pistelli, Berardi, Risi, Giotta, Lorusso, Rinaldi, Artale, Cazzaniga, Zustovich, Cappuzzo, Landi, Torrisi, Scagnoli, Botticelli, Michelotti, Fratini, Saltarelli, Paris, Muratore, Cassano, Gianni, Gaspari, Veltri, Zoratto, Fiorio, Fabbri, Mazzotta, Ruggeri, Pedersini, Valerio, Filomeno, Minelli, Scavina, Raffaele, Astone, De Vita, Pozzi, Riccardi, Greco, Moscetti, Giordano, Maugeri-Saccà, Zennaro, Botti, Pelle, Cappelli, Cavicchi, Vizza, Sanguineti, Tomao, Cortesi, Marchetti, Tomao, Speranza, Sperduti, Ciliberto and Vici.
Conflict of interest statement
LP received speaker fees from Novartis, outside the submitted work. UDG: Pfizer, BMS, MSD, PharmaMAR, AStellas, Bayer, Ipsen, Novartis; Invited speaker Roche, BMS, SAnofi, AstraZeneca; received research grants from AstraZeneca, SAnofi, Roche, outside the submitted work. AF received honoraria as a speaker from Eli Lilly, Novartis, Pierre-Fabre, outside the submitted work. GT: advisory boards from Novartis, Pfizer, Eisai, Roche, and Eli Lilly, outside the submitted work. DS: advisory boards from Novartis, Pfizer, Eisai, Roche, and Eli Lilly, outside the submitted work. NLV: Roche, MSD, Eisai, Novartis, AstraZeneca, GSK, Pfizer, Gentili, Daiichi Sankyo, Dephaforum, outside the submitted work. OG: Eisai, MSD, Gilead, Seagen, Novartis, Eli Lilly, outside the submitted work. IM: advisory boards from Eli Lilly, Novartis, Gentili, Roche, Pfizer, Ipsen, and Pierre-Fabre, outside the submitted work. GD’A: Novartis, Amgen, Eli Lilly outside the submitted work. TG received travel grants from Eisai, Roche, Pfizer, and Novartis; speaker fees/advisory boards from Roche, Pfizer, Novartis, Gentili, and Eli Lilly, outside the submitted work. MPi Consultant/advisory boards from Gilead, Eli Lilly, Pfizer, Novartis, Gentili, MSD, outside the submitted work. RB received research grant/advisory boards from AstraZeneca, Boehringer Ingelheim, Novartis, MSD, Otsuka, Eli Lilly, Roche, Amgen, GSK, Eisai, outside the submitted work. FGi: advisory boards from Gilead, Daiichi Sankyo, Seagen, outside the submitted work. MEC consultant/advisory role for Pierre-Fabre, Roche, Novartis, Eli Lilly, Celgene, outside the submitted work. RT: AstraZeneca, Eisai, Pfizer, Eli Lilly, MSD, Exact Science, outside the submitted work. AB: MSD, BMS, Pfizer, Novartis, Roche outside the submitted work. AM received travel grants from Eisai, Celgene, and Novartis Ipsen; personal fees/advisory boards from Eisai, Novartis, AstraZeneca, Teva, Pfizer, and Celgene, outside the submitted work. IP received personal fees/advisory boards from Roche, Pfizer, Novartis, Italfarmaco, Gentili, and Pierre-Fabre. LG received congress travel accomodation from Roche, Daiichi Sankyo, AstraZeneca, Pfizer, Novartis; advisory role for Astra Zeneca outside the submitted work. MMin: Novartis, MSD, Eli Lilly, outside the submitted work. LM received personal fees/advisory board from Roche, Novartis, Eisai, and Pfizer, outside the submitted work. EC: Astellas, Roche, BMS, Jansen, MSD, Sirtex, Merck, Bayer, Servier, Novartis, outside the submitted work. PM has/had a consultant/advisory role for BMS, Roche, Genentech, MSD, Novartis, Amgen, Merck Serono, Pierre-Fabre and Incyte, outside the submitted work. PV received speaker fees/advisory boards from Roche, Pfizer, Novartis and Eli Lilly, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

