Asymptomatic hyperuricaemia in chronic kidney disease: mechanisms and clinical implications
- PMID: 37261000
- PMCID: PMC10229286
- DOI: 10.1093/ckj/sfad006
Asymptomatic hyperuricaemia in chronic kidney disease: mechanisms and clinical implications
Abstract
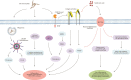
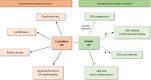
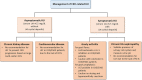
Asymptomatic hyperuricaemia (HU) is considered a pathogenic factor in multiple disease contexts, but a causative role is only proven for the crystalline form of uric acid in gouty arthritis and urate nephropathy. Epidemiological studies document a robust association of HU with hypertension, cardiovascular disease (CVD) and CKD progression, but CKD-related impaired uric acid (UA) clearance and the use of diuretics that further impair UA clearance likely accounts for these associations. Interpreting the available trial evidence is further complicated by referring to xanthine oxidase inhibitors as urate-lowering treatment, although these drugs inhibit other substrates, so attributing their effects only to HU is problematic. In this review we provide new mechanistic insights into the biological effects of soluble and crystalline UA and discuss clinical evidence on the role of asymptomatic HU in CKD, CVD and sterile inflammation. We identify research areas with gaps in experimental and clinical evidence, specifically on infectious complications that represent the second common cause of death in CKD patients, referred to as secondary immunodeficiency related to kidney disease. In addition, we address potential therapeutic approaches on how and when to treat asymptomatic HU in patients with kidney disease and where further interventional studies are required.
Keywords: asymptomatic hyperuricemia; cardiovascular disease; chronic kidney disease; gout; infection; uric acid.
© The Author(s) 2023. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
S.S. received funding from Eleva. H.J.A. received consultancy or lecture fees from Boehringer Ingelheim, Bayer, GlaxoSmithKline, AstraZeneca, Novartis, Otsuka, Janssen, Kezar, Sanofi, Vifor, Keza, Variant Bio and PreviPharma. Q.L. declares no conflicts of interest.
Figures

Similar articles
-
[URATE AS A POTENTIAL RISK FACTOR OF CARDIOVASCULAR AND RENAL DISEASES].Acta Med Croatica. 2016 Dec;70(4-5):233-9. Acta Med Croatica. 2016. PMID: 29087102 Review. Croatian.
-
Dotinurad: a novel selective urate reabsorption inhibitor as a future therapeutic option for hyperuricemia.Clin Exp Nephrol. 2020 Mar;24(Suppl 1):1-5. doi: 10.1007/s10157-019-01811-9. Epub 2019 Nov 21. Clin Exp Nephrol. 2020. PMID: 31754883 Free PMC article. Review.
-
Impact of hyperuricemia on chronic kidney disease and atherosclerotic cardiovascular disease.Hypertens Res. 2022 Apr;45(4):635-640. doi: 10.1038/s41440-021-00840-w. Epub 2022 Jan 19. Hypertens Res. 2022. PMID: 35046512 Review.
-
Uric acid and chronic kidney disease: which is chasing which?Nephrol Dial Transplant. 2013 Sep;28(9):2221-8. doi: 10.1093/ndt/gft029. Epub 2013 Mar 29. Nephrol Dial Transplant. 2013. PMID: 23543594 Free PMC article. Review.
-
Uric Acid as a Risk Factor for Chronic Kidney Disease and Cardiovascular Disease - Japanese Guideline on the Management of Asymptomatic Hyperuricemia.Circ J. 2021 Jan 25;85(2):130-138. doi: 10.1253/circj.CJ-20-0406. Epub 2020 Dec 18. Circ J. 2021. PMID: 33342914
Cited by
-
Association of urinary excretion rates of uric acid with biomarkers of kidney injury in patients with advanced chronic kidney disease.PLoS One. 2024 Jun 11;19(6):e0304105. doi: 10.1371/journal.pone.0304105. eCollection 2024. PLoS One. 2024. PMID: 38861521 Free PMC article.
-
Association between the combination of the triglyceride-glucose index and obesity-related indices with hyperuricemia among children and adolescents in China.Lipids Health Dis. 2025 Apr 23;24(1):150. doi: 10.1186/s12944-025-02547-0. Lipids Health Dis. 2025. PMID: 40269945 Free PMC article.
-
Increased risk of chronic kidney disease in uric acid stone formers with high neutrophil-to-lymphocyte ratio.Sci Rep. 2023 Oct 17;13(1):17686. doi: 10.1038/s41598-023-45034-1. Sci Rep. 2023. PMID: 37848540 Free PMC article.
-
Hyperuricemia-induced complications: dysfunctional macrophages serve as a potential bridge.Front Immunol. 2025 Jan 28;16:1512093. doi: 10.3389/fimmu.2025.1512093. eCollection 2025. Front Immunol. 2025. PMID: 39935474 Free PMC article. Review.
-
Tanshinone IIA Regulates NRF2/NLRP3 Signal Pathway to Restrain Oxidative Stress and Inflammation in Uric Acid-Induced HK-2 Fibrotic Models.Endocr Metab Immune Disord Drug Targets. 2025;25(9):721-731. doi: 10.2174/0118715303315786240926075342. Endocr Metab Immune Disord Drug Targets. 2025. PMID: 39473254
References
-
- Jager KJ, Kovesdy C, Langham Ret al. . A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int 2019;96:1048–50. - PubMed
-
- Vanholder R, De Smet R, Glorieux Get al. . Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int 2003;63:1934–43. - PubMed
-
- Vitart V, Rudan I, Hayward Cet al. . SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 2008;40:437–42. - PubMed
-
- Enomoto A, Kimura H, Chairoungdua Aet al. . Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002;417:447–52. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

