Aerosol vaccination of chicken pullets with irradiated avian pathogenic Escherichia coli induces a local immunostimulatory effect
- PMID: 37261344
- PMCID: PMC10227613
- DOI: 10.3389/fimmu.2023.1185232
Aerosol vaccination of chicken pullets with irradiated avian pathogenic Escherichia coli induces a local immunostimulatory effect
Abstract
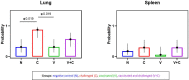
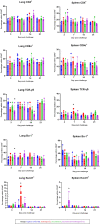
The present study investigated the expression of cytokines and cellular changes in chickens following vaccination with irradiated avian pathogenic Escherichia coli (APEC) and/or challenge. Four groups of 11-week-old pullets, each consisting of 16 birds were kept separately in isolators before they were sham inoculated (N), challenged only (C), vaccinated (V) or vaccinated and challenged (V+C). Vaccination was performed using irradiated APEC applied via aerosol. For challenge, the homologous strain was administered intratracheally. Birds were sacrificed on 3, 7, 14 and 21 days post challenge (dpc) to examine lesions, organ to body weight ratios and bacterial colonization. Lung and spleen were sampled for investigating gene expression of cytokines mediating inflammation by RT-qPCR and changes in the phenotype of subsets of mononuclear cells by flow cytometry. After re-stimulation of immune cells by co-cultivation with the pathogen, APEC-specific IFN-γ producing cells were determined. Challenged only birds showed more severe pathological and histopathological lesions, a higher probability of bacterial re-isolation and higher organ to body weight ratios compared to vaccinated and challenged birds. In the lung, an upregulation of IL-1β and IL-6 following vaccination and/or challenge at 3 dpc was observed, whereas in the spleen IL-1β was elevated. Changes were observed in macrophages and TCR-γδ+ cells within 7 dpc in spleen and lung of challenged birds. Furthermore, an increase of CD4+ cells in spleen and a rise of Bu-1+ cells in lung were present in vaccinated and challenged birds at 3 dpc. APEC re-stimulated lung and spleen mononuclear cells from only challenged pullets showed a significant increase of IFN-γ+CD8α+ and IFN-γ+TCR-γδ+ cells. Vaccinated and challenged chickens responded with a significant increase of IFN-γ+CD8α+ T cells in the lung and IFN-γ+TCR-γδ+ cells in the spleen. Re-stimulation of lung mononuclear cells from vaccinated birds resulted in a significant increase of both IFN-γ+CD8α+ and IFN-γ+TCR-γδ+ cells. In conclusion, vaccination with irradiated APEC caused enhanced pro-inflammatory response as well as the production of APEC-specific IFN-γ-producing γδ and CD8α T cells, which underlines the immunostimulatory effect of the vaccine in the lung. Hence, our study provides insights into the underlying immune mechanisms that account for the defense against APEC.
Keywords: APEC; T cells; chicken; colibacillosis; cytokine; immune response; interferon gamma.
Copyright © 2023 Bagheri, Mitra, Paudel, Abdelhamid, Könnyü, Wijewardana, Kangethe, Cattoli, Lyrakis, Hess, Hess and Liebhart.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Palygorskite improves growth performance and prevents liver damage in avian pathogenic Escherichia coli-challenged broiler chickens at an early age.J Anim Sci. 2024 Jan 3;102:skae302. doi: 10.1093/jas/skae302. J Anim Sci. 2024. PMID: 39373204
-
Production of interferon gamma and interleukin 17A in chicken T-cell subpopulations hallmarks the stimulation with live, irradiated and killed avian pathogenic Escherichia coli.Dev Comp Immunol. 2022 Aug;133:104408. doi: 10.1016/j.dci.2022.104408. Epub 2022 Apr 4. Dev Comp Immunol. 2022. PMID: 35390358
-
Intestinal differential metabolite tryptophan from avian pathogenic Escherichia coli (APEC)-resistant and susceptible chickens alleviates APEC symptoms in chickens.Poult Sci. 2025 Jul;104(7):105212. doi: 10.1016/j.psj.2025.105212. Epub 2025 Apr 24. Poult Sci. 2025. PMID: 40315585 Free PMC article.
-
Prevalence of specific serogroups, antibiotic resistance and virulence factors of avian pathogenic Escherichia coli (APEC) isolated from clinical cases: A systematic review and meta-analysis.Microb Pathog. 2024 Sep;194:106843. doi: 10.1016/j.micpath.2024.106843. Epub 2024 Aug 6. Microb Pathog. 2024. PMID: 39117015
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
Cited by
-
A comprehensive study of colisepticaemia progression in layer chickens applying novel tools elucidates pathogenesis and transmission of Escherichia coli into eggs.Sci Rep. 2024 Apr 6;14(1):8111. doi: 10.1038/s41598-024-58706-3. Sci Rep. 2024. PMID: 38582950 Free PMC article.
-
Cytokine and immunoglobulin profiles of Arbor Acres broiler chickens at different stages of physiological development.Vet World. 2024 May;17(5):988-993. doi: 10.14202/vetworld.2024.988-993. Epub 2024 May 4. Vet World. 2024. PMID: 38911092 Free PMC article.
References
-
- Nolan LK, Vaillancourt J-P, Barbieri NL, Logue CM. Colibacillosis. In: Swayne DE, Boulianne M, Logue CM, McDougald LR, Nair V, Suarez DL, et al., editors. Diseases of poultry, 14th. Hoboken, NJ: John Wiley & Sons, Inc; (2019). p. 770–830.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

