Efficacy and Safety of Eliapixant in Refractory Chronic Cough: The Randomized, Placebo-Controlled Phase 2b PAGANINI Study
- PMID: 37261531
- PMCID: PMC10234230
- DOI: 10.1007/s00408-023-00621-x
Efficacy and Safety of Eliapixant in Refractory Chronic Cough: The Randomized, Placebo-Controlled Phase 2b PAGANINI Study
Abstract
Introduction: The PAGANINI study evaluated the efficacy and safety of the selective P2X3 antagonist eliapixant in patients with refractory chronic cough (RCC).
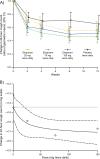
Methods: PAGANINI was a randomized, double-blind, parallel-group, placebo-controlled, multicenter, dose-finding, phase 2b study. Adults with RCC lasting ≥ 12 months and cough severity ≥ 40 mm on a visual analog scale at screening were enrolled. Participants were randomized 1:1:1:1 to twice-daily 25 mg, 75 mg, or 150 mg oral eliapixant or placebo for 12 weeks. The primary endpoint was change from baseline in 24-h cough count after 12 weeks of intervention.
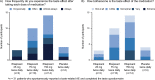
Results: Overall, 310 participants were randomized to twice-daily eliapixant 25 mg (n = 75), 75 mg (n = 78), 150 mg (n = 80), or placebo (n = 77). A statistically significant dose-response signal with eliapixant was detected for the primary endpoint (all dose-response models, adjusted p < 0.1; one-sided). Adverse events (AEs) were reported in 39 (51%) participants with placebo and 43-51 (57-65%) participants receiving eliapixant. The most common AE was dysgeusia, occurring in 1% (n = 1) of the placebo group and 1-16% (n = 1-13) of the eliapixant groups in a dose-related manner. One case of a moderate drug-induced liver injury occurred in a participant receiving 150 mg twice-daily eliapixant.
Conclusion: Eliapixant demonstrated efficacy and a favorable taste tolerability profile in RCC. However, a drug-induced liver injury contributed to intensified liver monitoring in clinical trials with eliapixant and discontinuation of the entire development program in all indications by Bayer AG.
Trial registration: ClinicalTrials.gov identifier NCT04562155; registered September 18, 2020.
Keywords: Chronic cough; Eliapixant; P2X3 receptor antagonist; Phase 2b clinical trial.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
PVD reports remuneration for consultancy from Bayer AG, Bellus Health, Chiesi, Merck, and Shionogi. AHM reports remuneration for consultancy from Bayer AG, Bellus Health, Boehringer Ingelheim, Merck, Pfizer, Procter & Gamble, and Shionogi, lecture fees from AstraZeneca and Boehringer Ingelheim, and grant support from Afferent Pharmaceuticals, Infirst, Merck, and Procter & Gamble. JAS reports research grants and remuneration for consultancy from Algernon, AstraZeneca, Axalbion, Bayer AG, Bellus Health, Boehringer Ingelheim, Chiesi, Merck, Nocion Therapeutics, Shionogi, and Trevi, and non-financial support and royalties from Vitalograph paid to Manchester University NHS Foundation Trust that may be shared with the department in which JAS works. JAS is funded by the Manchester NIHR Biomedical Research Centre and an NIHR Senior Investigator Award. MRS reports research grants from Afferent Pharmaceuticals, Bayer AG, Bellus Health, Merck, NeRRe Therapeutics, and Shionogi, remuneration for lectures from Merck, and has served on advisory committees for Afferent Pharmaceuticals, AstraZeneca, Bayer AG, Bellus Health, Merck, NeRRe Therapeutics, Nocion Therapeutics, and Shionogi and on a Data and Safety Monitoring Board for Nocion Therapeutics. MV reports remuneration for consultancy in litigation relating to acid suppressive therapy, has served on an advisory committee for Bayer AG, Diversatek, Ironwood, ISOThrive, Medtronic, Phathom, and Sanofi, and holds a patent with Diversatek. LG reports a research grant from AstraZeneca, remuneration for lectures from AstraZeneca, Bayer AG, GlaxoSmithKline, MSD, Novartis, and Sanofi, and remuneration for consultancy from AstraZeneca, Bayer AG, Chiesi, GlaxoSmithKline, Merck, MSD, Novartis, and Sanofi. AN reports research grants from Kyorin Pharmaceutical and Novartis and remuneration for lectures from AstraZeneca, GlaxoSmithKline, Kyorin Pharmaceutical, MSD, and Novartis. KG, UK, RS, and MW are employees of Bayer AG. PVP was an employee of Bayer AG at the time of the study and is now an employee of the Janssen Pharmaceutical Companies of Johnson & Johnson. LMG reports research grants from Bayer AG, Bellus Health, Chiesi, Merck, and Shionogi, remuneration for lectures from Bayer AG, Bellus Health, Chiesi, GlaxoSmithKline, Merck, and Shionogi, remuneration for consultancy from Bayer AG, Bellus Health, Chiesi, Merck, NeRRe Therapeutics, Nocion Therapeutics, and Shionogi, and has served on advisory committees for Applied Clinical Intelligence, Bayer AG, Bellus Health, Chiesi, Merck, NeRRe Therapeutics, Nocion Therapeutics, and Shionogi and on a Data and Safety Monitoring Board for Bayer AG.
Figures

References
-
- Morice AH, Millqvist E, Bieksiene K, Birring SS, Dicpinigaitis P, Domingo Ribas C, Hilton Boon M, Kantar A, Lai K, McGarvey L, Rigau D, Satia I, Smith J, Song WJ, Tonia T, van den Berg JWK, van Manen MJG, Zacharasiewicz A. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55(1):1901136. doi: 10.1183/13993003.01136-2019. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

