Camrelizumab Plus Apatinib and Temozolomide as First-Line Treatment in Patients With Advanced Acral Melanoma: The CAP 03 Phase 2 Nonrandomized Clinical Trial
- PMID: 37261804
- PMCID: PMC10236335
- DOI: 10.1001/jamaoncol.2023.1363
Camrelizumab Plus Apatinib and Temozolomide as First-Line Treatment in Patients With Advanced Acral Melanoma: The CAP 03 Phase 2 Nonrandomized Clinical Trial
Abstract
Importance: Acral melanoma, known for low tumor mutation burden, responds poorly to immunotherapy. A standard therapy is still lacking.
Objective: To investigate the activity and safety of camrelizumab (an anti-programmed cell death-1 antibody) plus apatinib (a vascular endothelial growth factor receptor 2 inhibitor) and temozolomide as first-line treatment in patients with advanced acral melanoma.
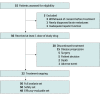
Design, setting, and participants: In this single-arm, single-center, phase 2 nonrandomized clinical trial, patients with treatment-naive unresectable stage III or IV acral melanoma were enrolled at Peking University Cancer Hospital and Institute between June 4, 2020, and August 24, 2021. The data cutoff date was April 10, 2022.
Interventions: Patients received 4-week cycles of intravenous camrelizumab, 200 mg, every 2 weeks; oral apatinib 250 mg, once daily; and intravenous temozolomide, 200 mg/m2, once daily on days 1 to 5 until disease progression or unacceptable toxic effects.
Main outcomes and measures: The primary end point was objective response rate as assessed by investigators according to the Response Evaluation Criteria In Solid Tumors (version 1.1). Secondary end points included progression-free survival, time to response, duration of response, disease control rate, overall survival, and safety.
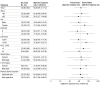
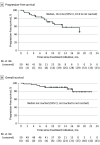
Results: A total of 50 patients (32 men [64%]; median age, 57 years [IQR, 52-62 years]) were enrolled and received treatment. The median follow-up duration was 13.4 months (IQR, 9.6-16.2 months). The objective response rate was 64.0% (32 of 50; 95% CI, 49.2%-77.1%). The median time to response and duration of response were 2.7 months (IQR, 0.9-2.9 months) and 17.5 months (95% CI, 12.0 to not reached), respectively. The disease control rate was 88.0% (44 of 50; 95% CI, 75.7%-95.5%). The estimated median progression-free survival was 18.4 months (95% CI, 10.6 to not reached). The median overall survival was not reached. The most common grade 3 or 4 treatment-related adverse events were increased gamma-glutamyltransferase levels (15 [30%]), decreased neutrophil count (11 [22%]), increased conjugated bilirubin levels (10 [20%]), and increased aspartate aminotransferase levels (10 [20%]). No treatment-related deaths occurred.
Conclusions and relevance: The findings of this nonrandomized clinical trial suggest that camrelizumab plus apatinib and temozolomide may be a potential first-line treatment option for patients with advanced acral melanoma, which warrants further validation in a randomized clinical trial.
Trial registration: ClinicalTrials.gov Identifier: NCT04397770.
Conflict of interest statement
Figures

References
-
- Newell F, Johansson PA, Wilmott JS, et al. . Comparative genomics provides etiologic and biological insight into melanoma subtypes. Cancer Discov. 2022;12(12):2856-2879. doi:10.1158/2159-8290.CD-22-0603 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

