Mucosal transcriptomics highlight lncRNAs implicated in ulcerative colitis, Crohn's disease, and celiac disease
- PMID: 37261910
- PMCID: PMC10443795
- DOI: 10.1172/jci.insight.170181
Mucosal transcriptomics highlight lncRNAs implicated in ulcerative colitis, Crohn's disease, and celiac disease
Abstract
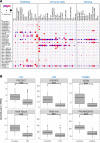
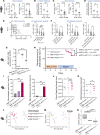
Ulcerative colitis (UC), Crohn's disease (CD), and celiac disease are prevalent intestinal inflammatory disorders with nonsatisfactory therapeutic interventions. Analyzing patient data-driven cohorts can highlight disease pathways and new targets for interventions. Long noncoding RNAs (lncRNAs) are attractive candidates, since they are readily targetable by RNA therapeutics, show relative cell-specific expression, and play key cellular functions. Uniformly analyzing gut mucosal transcriptomics from 696 subjects, we have highlighted lncRNA expression along the gastrointestinal (GI) tract, demonstrating that, in control samples, lncRNAs have a more location-specific expression in comparison with protein-coding genes. We defined dysregulation of lncRNAs in treatment-naive UC, CD, and celiac diseases using independent test and validation cohorts. Using the Predicting Response to Standardized Pediatric Colitis Therapy (PROTECT) inception UC cohort, we defined and prioritized lncRNA linked with UC severity and prospective outcomes, and we highlighted lncRNAs linked with gut microbes previously implicated in mucosal homeostasis. HNF1A-AS1 lncRNA was reduced in all 3 conditions and was further reduced in more severe UC form. Similarly, the reduction of HNF1A-AS1 ortholog in mice gut epithelia showed higher sensitivity to dextran sodium sulfate-induced colitis, which was coupled with alteration in the gut microbial community. These analyses highlight prioritized dysregulated lncRNAs that can guide future preclinical studies for testing them as potential targets.
Keywords: Gastroenterology; Inflammatory bowel disease.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

