New perspectives in diabetic neuropathy
- PMID: 37263266
- PMCID: PMC10525009
- DOI: 10.1016/j.neuron.2023.05.003
New perspectives in diabetic neuropathy
Abstract
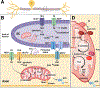
Diabetes prevalence continues to climb with the aging population. Type 2 diabetes (T2D), which constitutes most cases, is metabolically acquired. Diabetic peripheral neuropathy (DPN), the most common microvascular complication, is length-dependent damage to peripheral nerves. DPN pathogenesis is complex, but, at its core, it can be viewed as a state of impaired metabolism and bioenergetics failure operating against the backdrop of long peripheral nerve axons supported by glia. This unique peripheral nerve anatomy and the injury consequent to T2D underpins the distal-to-proximal symptomatology of DPN. Earlier work focused on the impact of hyperglycemia on nerve damage and bioenergetics failure, but recent evidence additionally implicates contributions from obesity and dyslipidemia. This review will cover peripheral nerve anatomy, bioenergetics, and glia-axon interactions, building the framework for understanding how hyperglycemia and dyslipidemia induce bioenergetics failure in DPN. DPN and painful DPN still lack disease-modifying therapies, and research on novel mechanism-based approaches is also covered.
Keywords: bioenergetics; diabetes; dyslipidemia; hyperglycemia; metabolic syndrome; mitochondria; obesity; pathophysiology; peripheral neuropathy; prediabetes.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.L.B. has acted as a consultant in the last 2 years for AditumBio, Amgen, Biointervene, Bristows, LatigoBio, GSK, Ionis, Lexicon therapeutics, Neuvati, Olipass, Orion, Replay, Third Rock Ventures, and Vida Ventures on behalf of Oxford University Innovation. D.L.B. has received research funding from Lilly and Astra Zeneca.
Figures



References
-
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et al. (2022). IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183, 109119. 10.1016/j.diabres.2021.109119. - DOI - PMC - PubMed
-
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, et al. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 157, 107843. 10.1016/j.diabres.2019.107843. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- F32 NS011024/NS/NINDS NIH HHS/United States
- MR/W002388/1/MRC_/Medical Research Council/United Kingdom
- R01 DK129320/DK/NIDDK NIH HHS/United States
- MR/M02394X/1/MRC_/Medical Research Council/United Kingdom
- R01 NS123450/NS/NINDS NIH HHS/United States
- K99 DK119366/DK/NIDDK NIH HHS/United States
- 202747/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MR/T020113/1/MRC_/Medical Research Council/United Kingdom
- P30 DK020572/DK/NIDDK NIH HHS/United States
- P30 DK063608/DK/NIDDK NIH HHS/United States
- R01 DK130913/DK/NIDDK NIH HHS/United States
- R01 NS111024/NS/NINDS NIH HHS/United States
- R00 DK119366/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

