A non-transmissible live attenuated SARS-CoV-2 vaccine
- PMID: 37263272
- PMCID: PMC10214529
- DOI: 10.1016/j.ymthe.2023.05.004
A non-transmissible live attenuated SARS-CoV-2 vaccine
Abstract
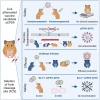
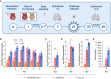
Live attenuated vaccines (LAVs) administered via the mucosal route may offer better control of the COVID-19 pandemic than non-replicating vaccines injected intramuscularly. Conceptionally, LAVs have several advantages, including presentation of the entire antigenic repertoire of the virus, and the induction of strong mucosal immunity. Thus, immunity induced by LAV could offer superior protection against future surges of COVID-19 cases caused by emerging SARS-CoV-2 variants. However, LAVs carry the risk of unintentional transmission. To address this issue, we investigated whether transmission of a SARS-CoV-2 LAV candidate can be blocked by removing the furin cleavage site (FCS) from the spike protein. The level of protection and immunity induced by the attenuated virus with the intact FCS was virtually identical to the one induced by the attenuated virus lacking the FCS. Most importantly, removal of the FCS completely abolished horizontal transmission of vaccine virus between cohoused hamsters. Furthermore, the vaccine was safe in immunosuppressed animals and showed no tendency to recombine in vitro or in vivo with a SARS-CoV-2 field strain. These results indicate that removal of the FCS from SARS-CoV-2 LAV is a promising strategy to increase vaccine safety and prevent vaccine transmission without compromising vaccine efficacy.
Keywords: COVID-19; SARS-CoV-2; live attenuated virus; mucosal vaccination; pneumonia; vaccine; virus transmission.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Related to this work, Freie Universität Berlin has filed a patent application for the use of sCPD9 and sCPD9-ΔFCS as vaccine. In this application, J.T., N.O., and D.K. are named as inventors of sCPD9. Freie Universität Berlin is collaborating with RocketVax AG for further development of sCPD9-ΔFCS as vaccine and receives funding for research.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

