A Medium-Chain Fatty Acid Analogue Prevents Intestinal Failure-Associated Liver Disease in Preterm Yorkshire Piglets
- PMID: 37263310
- PMCID: PMC10527514
- DOI: 10.1053/j.gastro.2023.05.035
A Medium-Chain Fatty Acid Analogue Prevents Intestinal Failure-Associated Liver Disease in Preterm Yorkshire Piglets
Abstract
Background & aims: At least 20%-30% of patients with intestinal failure receiving long-term parenteral nutrition will develop intestinal failure-associated liver disease (IFALD), for which there are few therapeutic options. SEFA-6179 is a first-in-class structurally engineered medium-chain fatty acid analogue that acts through GPR84, PPARα, and PPARγ agonism. We hypothesized that SEFA-6179 would prevent biochemical and histologic liver injury in a preterm piglet model of IFALD.
Methods: Preterm Yorkshire piglets were delivered by cesarean section, and parenteral nutrition was provided for 14 days via implanted central venous catheters. Animals were treated with either medium-chain triglyceride vehicle control or SEFA-6179.
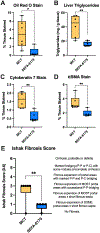
Results: Compared to medium-chain triglyceride vehicle at day of life 15, SEFA-6179 prevented biochemical cholestasis (direct bilirubin: 1.9 vs <0.2 mg/dL, P = .01; total bilirubin: 2.7 vs 0.4 mg/dL, P = .02; gamma glutamyl transferase: 172 vs 30 U/L, P = .01). SEFA-6179 also prevented steatosis (45.6 vs 13.9 mg triglycerides/g liver tissue, P = .009), reduced bile duct proliferation (1.6% vs 0.5% area cytokeratin 7 positive, P = .009), and reduced fibrosis assessed by a masked pathologist (median Ishak score: 3 vs 1, P = 0.007). RNA sequencing of liver tissue demonstrated that SEFA-6179 broadly impacted inflammatory, metabolic, and fibrotic pathways, consistent with its in vitro receptor activity (GPR84/PPARα/PPARγ agonist).
Conclusions: In a preterm piglet model of IFALD, SEFA-6179 treatment prevented biochemical cholestasis and steatosis and reduced bile duct proliferation and fibrosis. SEFA-6179 is a promising first-in-class therapy for the prevention and treatment of IFALD that will be investigated in an upcoming phase II clinical trial.
Keywords: Animal; Cholestasis; Intestinal Failure; Liver Diseases/Drug Therapy; Models; Parenteral Nutrition.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
This study was primarily funded via a sponsored research agreement with NorthSea Therapeutics. Dr. Fraser is the Chief Scientific Officer of NorthSea Therapeutics and was responsible for the
Figures





References
-
- Lauriti G, Zani A, Aufieri R, et al. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J Parenter Enteral Nutr 2014;38:70–85. - PubMed
-
- Christensen RD, Henry E, Wiedmeier SE, et al. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J Perinatol 2007;27:284–290. - PubMed
-
- Lee WS, Chew KS, Ng RT, et al. Intestinal failure-associated liver disease (IFALD): insights into pathogenesis and advances in management. Hepatol Int 2020;14:305–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

