Off-pump injectable versus on-pump conventional tissue valves for pulmonary valve replacement: the injectable valve implantation randomised trial (INVITE)
- PMID: 37263697
- PMCID: PMC10254783
- DOI: 10.1136/bmjopen-2022-065192
Off-pump injectable versus on-pump conventional tissue valves for pulmonary valve replacement: the injectable valve implantation randomised trial (INVITE)
Abstract
Objectives: To assess the effectiveness of injectable tissue pulmonary valve compared with standard pulmonary valve in patients requiring pulmonary valve replacement surgery.
Design: A multicentre, single-blind, parallel two-group randomised controlled trial. Participants were blind to their allocation. Follow-up continued for 6 months. Randomised allocations were generated by a computer using block randomisation, stratified by centre.
Setting: Two National Health Service secondary care centres in the UK.
Participants: People aged 12-80 years requiring pulmonary valve replacement.
Interventions: Participants were randomly allocated (1:1 ratio) to injectable pulmonary valve replacement (IPVR) without cardiopulmonary bypass (CPB) or standard pulmonary valve replacement (SPVR) with CPB.
Primary and secondary outcome measures: The primary outcome was chest drainage volume over the first 24 hours after surgery. Secondary outcomes included in-hospital clinical outcomes; valve and heart function 6 months postsurgery and health-related quality of life 6 weeks and 6 months postsurgery.
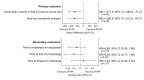
Results: Nineteen participants agreed to take part. Eleven were allocated to IPVR and eight to SPVR. The trial was stopped before the target sample size of 60 participants was reached due to challenges in recruitment. The primary analysis includes all randomised participants; there were no withdrawals. Chest drain volume 24 hours after surgery was on average 277.6 mL lower with IPVR (IPVR mean 340.0 mL; SPVR mean 633.8 mL; mean difference, -277.6; 95% CI, -484.0 to -71.2; p=0.005). There were no statistically significant differences in time to readiness for extubation (p=0.476), time to fitness for discharge (p=0.577) and time to first discharge from the intensive care unit (p=0.209). Six participants with IPVR required CPB. Safety profiles and quality of life scores were similar.
Conclusions: IPVR reduced chest drain volume despite >50% of participants requiring CPB. There was no evidence of any other benefit of IPVR.
Trial registration number: ISRCTN23538073.
Keywords: Cardiac surgery; Cardiothoracic surgery; Clinical trials; Paediatric cardiac surgery.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Pierson Ltd agreed to provide the injectable tissue valves (BioPulmonic, Biointegral Surgical) used in the trial at a discounted rate. Pierson Ltd paid for travel-related expenses for Stefano Marianeschi to visit the trial centres and provide injectable pulmonary valve replacement training.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
