ALL-RIC trial protocol: a comparison of reduced dose total body irradiation (TBI) and cyclophosphamide with fludarabine and melphalan reduced intensity conditioning in adults with acute lymphoblastic leukaemia (ALL) in complete remission
- PMID: 37263700
- PMCID: PMC10255288
- DOI: 10.1136/bmjopen-2022-067790
ALL-RIC trial protocol: a comparison of reduced dose total body irradiation (TBI) and cyclophosphamide with fludarabine and melphalan reduced intensity conditioning in adults with acute lymphoblastic leukaemia (ALL) in complete remission
Abstract
Introduction: The usage of a T-cell depleted, reduced intensity conditioning (RIC) approach to haematopoietic cell transplantation (HCT) in adult patients with acute lymphoblastic leukaemia (ALL) over 40 years of age and in first complete remission (CR) has resulted in encouraging rates of event-free and overall survival in a population of adults with high risk disease. However, relapse rates remain high-with disease progression being the major cause of treatment failure. Using different, more powerful conditioning approaches is the logical next step in examining the role of RIC allogeneic HCT in adult ALL.
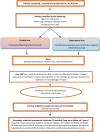
Methods and analysis: The ALL-RIC trial is a two-arm, phase II, multicentre, randomised clinical trial in adult patients with ALL in first or second CR, who are undergoing allogeneic HCT. Comparison of a novel RIC transplant conditioning regimen using reduced-dose total body irradiation (TBI), cyclophosphamide and alemtuzumab, is made against a standardised RIC approach using fludarabine, melphalan and alemtuzumab. The primary outcome of the study is disease-free survival at 3 years, defined as time from randomisation to the first of either relapse or death from any cause. Patients who are still alive and progression-free at the end of the trial will be censored at their last date known to be alive. Secondary outcomes include overall survival and non-relapse mortality.
Ethics and dissemination: The protocol was approved by the East Midlands-Leicester Central Research Ethics committee (18/EM/0112). Initial approval was received on 12 June 2018. Current protocol version (V.6.0) approval obtained on 18 November 2019. The Medicines and Healthcare products Regulatory Agency (MHRA) also approved all protocol versions. The results of this trial will be disseminated through national and international presentations and peer-reviewed publications.
Trial registration number: EudraCT Number: 2017-004800-23.ISRCTN99927695.
Keywords: Bone marrow transplantation; Leukaemia; Transplant medicine.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Marks DI, Clifton-Hadley L, Copland M, et al. . In-vivo T-cell depleted reduced-intensity conditioned allogeneic haematopoietic stem-cell transplantation for patients with acute Lymphoblastic leukaemia in first remission: results from the prospective, single-arm evaluation of the Ukall14 trial. Lancet Haematol 2022;9:e276–88. 10.1016/S2352-3026(22)00036-9 - DOI - PMC - PubMed
-
- Okasha D, Kirkwood AA, Copland M, et al. . Fludarabine, Melphalan and Alemtuzumab conditioned reduced intensity (RIC) allogeneic hematopoietic cell transplantation for adults aged >40 years with de novo acute Lymphoblastic leukemia: A prospective study from the Ukall14 trial [ISRCTN 66541317]. Blood 2015;126:733. 10.1182/blood.V126.23.733.733 - DOI - PubMed
-
- Marks DI, Wang T, Pérez WS, et al. . The outcome of full-intensity and reduced-intensity conditioning matched Sibling or unrelated donor transplantation in adults with Philadelphia Chromosome-negative acute Lymphoblastic leukemia in first and second complete remission. Blood 2010;116:366–74. 10.1182/blood-2010-01-264077 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources