Automatic AI-based contouring of prostate MRI for online adaptive radiotherapy
- PMID: 37263911
- PMCID: PMC11156783
- DOI: 10.1016/j.zemedi.2023.05.001
Automatic AI-based contouring of prostate MRI for online adaptive radiotherapy
Abstract
Background and purpose: MR-guided radiotherapy (MRgRT) online plan adaptation accounts for tumor volume changes, interfraction motion and thus allows daily sparing of relevant organs at risk. Due to the high interfraction variability of bladder and rectum, patients with tumors in the pelvic region may strongly benefit from adaptive MRgRT. Currently, fast automatic annotation of anatomical structures is not available within the online MRgRT workflow. Therefore, the aim of this study was to train and validate a fast, accurate deep learning model for automatic MRI segmentation at the MR-Linac for future implementation in a clinical MRgRT workflow.
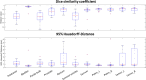
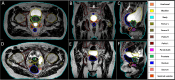
Materials and methods: For a total of 47 patients, T2w MRI data were acquired on a 1.5 T MR-Linac (Unity, Elekta) on five different days. Prostate, seminal vesicles, rectum, anal canal, bladder, penile bulb, body and bony structures were manually annotated. These training data consisting of 232 data sets in total was used for the generation of a deep learning based autocontouring model and validated on 20 unseen T2w-MRIs. For quantitative evaluation the validation set was contoured by a radiation oncologist as gold standard contours (GSC) and compared in MATLAB to the automatic contours (AIC). For the evaluation, dice similarity coefficients (DSC), and 95% Hausdorff distances (95% HD), added path length (APL) and surface DSC (sDSC) were calculated in a caudal-cranial window of ± 4 cm with respect to the prostate ends. For qualitative evaluation, five radiation oncologists scored the AIC on the possible usage within an online adaptive workflow as follows: (1) no modifications needed, (2) minor adjustments needed, (3) major adjustments/ multiple minor adjustments needed, (4) not usable.
Results: The quantitative evaluation revealed a maximum median 95% HD of 6.9 mm for the rectum and minimum median 95% HD of 2.7 mm for the bladder. Maximal and minimal median DSC were detected for bladder with 0.97 and for penile bulb with 0.73, respectively. Using a tolerance level of 3 mm, the highest and lowest sDSC were determined for rectum (0.94) and anal canal (0.68), respectively. Qualitative evaluation resulted in a mean score of 1.2 for AICs over all organs and patients across all expert ratings. For the different autocontoured structures, the highest mean score of 1.0 was observed for anal canal, sacrum, femur left and right, and pelvis left, whereas for prostate the lowest mean score of 2.0 was detected. In total, 80% of the contours were rated be clinically acceptable, 16% to require minor and 4% major adjustments for online adaptive MRgRT.
Conclusion: In this study, an AI-based autocontouring was successfully trained for online adaptive MR-guided radiotherapy on the 1.5 T MR-Linac system. The developed model can automatically generate contours accepted by physicians (80%) or only with the need of minor corrections (16%) for the irradiation of primary prostate on the clinically employed sequences.
Keywords: Adaptive radiotherapy; Automatic annotations; Deep learning; MR-Linac; MR-only.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [The authors report institutional collaborations with TheraPanacea, Elekta, Philips, Kaiku and PTW Freiburg which provided technical and/or financial support. TheraPanacea developed the proposed model, but were not involved in the analysis and scoring of the testing dataset.]
Figures


References
-
- Christiansen R.L., Dysager L., Hansen C.R., Jensen H.R., Schytte T., Nyborg C.J., Bertelsen A.S., Agergaard S.N., Mahmood F., Hansen S., Hansen O., Brink C., Bernchou U. Online adaptive radiotherapy potentially reduces toxicity for high-risk prostate cancer treatment. Radiother Oncol. 2022;167:165–171. doi: 10.1016/j.radonc.2021.12.013. - DOI - PubMed
-
- Nachbar M., Mönnich D., Boeke S., Gani C., Weidner N., Heinrich V., Lo Russo M., Livi L., Winter J., Tsitsekidis S., Dohm O., Thorwarth D., Zips D., De-Colle C. Partial breast irradiation with the 1.5 T MR-Linac: First patient treatment and analysis of electron return and stream effects. Radiother Oncol. 2019;145:30–35. doi: 10.1016/j.radonc.2019.11.025. - DOI - PubMed
-
- Thorwarth D., Ege M., Nachbar M., Mönnich D., Gani C., Zips D., Boeke S. Quantitative magnetic resonance imaging on hybrid magnetic resonance linear accelerators: Perspective on technical and clinical validation. Phys Imag Radiat Oncol. 2020;16:69–73. doi: 10.1016/j.phro.2020.09.007. - DOI - PMC - PubMed
-
- Gani C., Lo Russo M., Boeke S., Wegener D., Gatidis S., Butzer S., Boldt J., Mönnich D., Thorwarth D., Nikolaou K., Zips D., Nachbar M. A novel approach for radiotherapy dose escalation in rectal cancer using online MR-guidance and rectal ultrasound gel filling - Rationale and first in human. Radiother Oncol. 2021;164:37–42. doi: 10.1016/j.radonc.2021.09.002. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

