Vagus nerve stimulation primes platelets and reduces bleeding in hemophilia A male mice
- PMID: 37264009
- PMCID: PMC10235098
- DOI: 10.1038/s41467-023-38505-6
Vagus nerve stimulation primes platelets and reduces bleeding in hemophilia A male mice
Abstract
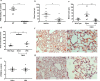
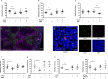
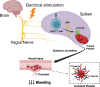
Deficiency of coagulation factor VIII in hemophilia A disrupts clotting and prolongs bleeding. While the current mainstay of therapy is infusion of factor VIII concentrates, inhibitor antibodies often render these ineffective. Because preclinical evidence shows electrical vagus nerve stimulation accelerates clotting to reduce hemorrhage without precipitating systemic thrombosis, we reasoned it might reduce bleeding in hemophilia A. Using two different male murine hemorrhage and thrombosis models, we show vagus nerve stimulation bypasses the factor VIII deficiency of hemophilia A to decrease bleeding and accelerate clotting. Vagus nerve stimulation targets acetylcholine-producing T lymphocytes in spleen and α7 nicotinic acetylcholine receptors (α7nAChR) on platelets to increase calcium uptake and enhance alpha granule release. Splenectomy or genetic deletion of T cells or α7nAChR abolishes vagal control of platelet activation, thrombus formation, and bleeding in male mice. Vagus nerve stimulation warrants clinical study as a therapy for coagulation disorders and surgical or traumatic bleeding.
© 2023. The Author(s).
Conflict of interest statement
J.M.H. and K.J.T. hold intellectual property rights and serve as consultants for Five Liters, Inc. C.J.C. holds intellectual property rights and is an employee at Five Liters, Inc. The remaining authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

