Combination of genetically diverse Pseudomonas phages enhances the cocktail efficiency against bacteria
- PMID: 37264114
- PMCID: PMC10235106
- DOI: 10.1038/s41598-023-36034-2
Combination of genetically diverse Pseudomonas phages enhances the cocktail efficiency against bacteria
Abstract
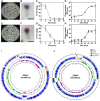
Phage treatment has been used as an alternative to antibiotics since the early 1900s. However, bacteria may acquire phage resistance quickly, limiting the use of phage treatment. The combination of genetically diverse phages displaying distinct replication machinery in phage cocktails has therefore become a novel strategy to improve therapeutic outcomes. Here, we isolated and studied lytic phages (SPA01 and SPA05) that infect a wide range of clinical Pseudomonas aeruginosa isolates. These relatively small myophages have around 93 kbp genomes with no undesirable genes, have a 30-min latent period, and reproduce a relatively high number of progenies, ranging from 218 to 240 PFU per infected cell. Even though both phages lyse their hosts within 4 h, phage-resistant bacteria emerge during the treatment. Considering SPA01-resistant bacteria cross-resist phage SPA05 and vice versa, combining SPA01 and SPA05 for a cocktail would be ineffective. According to the decreased adsorption rate of the phages in the resistant isolates, one of the anti-phage mechanisms may occur through modification of phage receptors on the target cells. All resistant isolates, however, are susceptible to nucleus-forming jumbophages (PhiKZ and PhiPA3), which are genetically distinct from phages SPA01 and SPA05, suggesting that the jumbophages recognize a different receptor during phage entry. The combination of these phages with the jumbophage PhiKZ outperforms other tested combinations in terms of bactericidal activity and effectively suppresses the emergence of phage resistance. This finding reveals the effectiveness of the diverse phage-composed cocktail for reducing bacterial growth and prolonging the evolution of phage resistance.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

