Early stage gastric adenocarcinoma: clinical and molecular landscapes
- PMID: 37264184
- PMCID: PMC11869628
- DOI: 10.1038/s41571-023-00767-w
Early stage gastric adenocarcinoma: clinical and molecular landscapes
Abstract
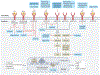
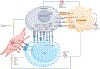
Gastric adenocarcinoma, even when diagnosed at an early (localized) disease stage, poses a major health-care burden with cure rates that remain unsatisfactorily low, particularly in Western countries. This lack of progress reflects, among other aspects, the impracticality of early diagnosis, considerable variations in therapeutic approaches that is partly based on regional preferences, and the ingrained heterogeneity of gastric adenocarcinoma cells and their associated tumour microenvironment (TME). Clinical trials have long applied empirical interventions with the assumption that all early stage gastric adenocarcinomas are alike. Despite certain successes, the shortcomings of these approaches can potentially be overcome by targeting the specific molecular subsets of gastric adenocarcinomas identified by genomic and/or multi-omics analyses, including microsatellite instability-high, Epstein-Barr virus-induced, DNA damage repair-deficient, HER2-positive and PD-L1-high subtypes. Future approaches, including the availability of sophisticated vaccines, novel antibody technologies, agents targeting TME components (including fibroblasts, macrophages, cytokines or chemokines, and T cells) and novel immune checkpoint inhibitors, supported by improved tissue-based and blood-based diagnostic assays, seem promising. In this Review, we highlight current knowledge of the molecular and cellular biology of gastric adenocarcinomas, summarize the current approaches to clinical management of the disease, and consider the role of novel management and/or treatment strategies.
© 2023. Springer Nature Limited.
Conflict of interest statement
J.A.A. has acted as an adviser to Aadi, Arcus, Amgen, Astellas, AZ, BMS, BeiGene, Daiichi, Gilead, Grail, Merck, Novartis, Servier and Zymeworks, and has received institutional research funding from Astellas, BMS, Daiichi, Delta Fly, Gilead, LaNova, Leap, Merck, Prolinx, Taiho, Turning Point and Zymeworks. The other authors declare no competing interests.
Figures



References
-
- Sung H et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 71, 209–249 (2021). - PubMed
-
- Parkin DM Global cancer statistics in the year 2000. Lancet Oncol 2, 533–543 (2001). - PubMed
-
- Cats A et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol 19, 616–628 (2018). - PubMed
-
- Bonenkamp JJ et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 345, 745–748 (1995). - PubMed
-
- Sakuramoto S et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med 357, 1810–1820 (2007). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

