Biomechanical control of lymphatic vessel physiology and functions
- PMID: 37264249
- PMCID: PMC10469203
- DOI: 10.1038/s41423-023-01042-9
Biomechanical control of lymphatic vessel physiology and functions
Abstract
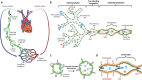
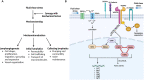
The ever-growing research on lymphatic biology has clearly identified lymphatic vessels as key players that maintain human health through their functional roles in tissue fluid homeostasis, immunosurveillance, lipid metabolism and inflammation. It is therefore not surprising that the list of human diseases associated with lymphatic malfunctions has grown larger, including issues beyond lymphedema, a pathology traditionally associated with lymphatic drainage insufficiency. Thus, the discovery of factors and pathways that can promote optimal lymphatic functions may offer new therapeutic options. Accumulating evidence indicates that aside from biochemical factors, biomechanical signals also regulate lymphatic vessel expansion and functions postnatally. Here, we review how mechanical forces induced by fluid shear stress affect the behavior and functions of lymphatic vessels and the mechanisms lymphatic vessels employ to sense and transduce these mechanical cues into biological signals.
Keywords: biomechanical force; human diseases; lymphatic vessel; mechanosensing; mechanotransduction.
© 2023. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Petrova TV, Koh GY. Biological functions of lymphatic vessels. Science. 2020;369. 10.1126/science.aax4063. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

