Feasibility of repairing skin defects by VEGF165 gene-modified iPS-HFSCs seeded on a 3D printed scaffold containing astragalus polysaccharide
- PMID: 37264501
- PMCID: PMC10399531
- DOI: 10.1111/jcmm.17800
Feasibility of repairing skin defects by VEGF165 gene-modified iPS-HFSCs seeded on a 3D printed scaffold containing astragalus polysaccharide
Abstract
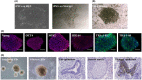
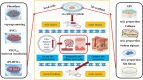
The preparation of biodegradable scaffolds loaded with cells and cytokine is a feature of tissue-engineered skin. IPSCs-based tissue-engineered skin treatment for wound repair is worth exploring. Healthy human skin fibroblasts were collected and reprogrammed into iPSCs. After gene modification and induction, CK19+ /Integrinβ1+ /CD200+ VEGF165 gene-modified iPS-HFSCsGFP were obtained and identified by a combination of immunofluorescence and RT-qPCR. Astragalus polysaccharide-containing 3D printed degradable scaffolds were prepared and co-cultured with VEGF165 gene-modified iPS-HFSCsGFP , and the biocompatibility and spatial structure of the tissue-engineered skin was analysed by cell counting kit-8 (CCK8) assay and scanning electron microscopy. Finally, the tissue-engineered skin was transplanted onto the dorsal trauma of nude mice, and the effect of tissue-engineered skin on the regenerative repair of total skin defects was evaluated by a combination of histology, immunohistochemistry, immunofluorescence, RT-qPCR, and in vivo three-dimensional reconstruction under two-photon microscopy. CK19+ /Integrinβ1+ /CD200+ VEGF165 gene-modified iPS-HFSCsGFP , close to the morphology and phenotype of human-derived hair follicle stem cells, were obtained. The surface of the prepared 3D printed degradable scaffold containing 200 μg/mL astragalus polysaccharide was enriched with honeycomb-like meshwork, which was more conducive to the proliferation of the resulting cells. After tissue-engineered skin transplantation, combined assays showed that it promoted early vascularization, collagen and hair follicle regeneration and accelerated wound repair. VEGF165 gene-modified iPS-HFSCsGFP compounded with 3D printed degradable scaffolds containing 200 μg/mL astragalus polysaccharide can directly and indirectly participate in vascular, collagen, and hair follicle regeneration in the skin, achieving more complete structural and functional skin regenerative repair.
Keywords: 3D printed degradable scaffold; astragalus polysaccharide; hair follicle stem cells; induced pluripotent stem cells; regeneration and repair; skin defect.
© 2023 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
All the authors declare that they have no conflicts of interest.
Figures





Similar articles
-
Astragalus polysaccharide-containing 3D-printed scaffold for traumatized skin repair and proteomic study.J Cell Mol Med. 2024 Aug;28(16):e70023. doi: 10.1111/jcmm.70023. J Cell Mol Med. 2024. PMID: 39158533 Free PMC article.
-
Gelatin-chondroitin-6-sulfate-hyaluronic acid scaffold seeded with vascular endothelial growth factor 165 modified hair follicle stem cells as a three-dimensional skin substitute.Stem Cell Res Ther. 2014 Oct 20;5(5):118. doi: 10.1186/scrt508. Stem Cell Res Ther. 2014. PMID: 25331352 Free PMC article.
-
Feasibility of repairing full-thickness skin defects by iPSC-derived epithelial stem cells seeded on a human acellular amniotic membrane.Stem Cell Res Ther. 2019 May 31;10(1):155. doi: 10.1186/s13287-019-1234-9. Stem Cell Res Ther. 2019. PMID: 31151466 Free PMC article.
-
Three-dimensional (3D) printed scaffold and material selection for bone repair.Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
-
Recent Advances in the Design of Three-Dimensional and Bioprinted Scaffolds for Full-Thickness Wound Healing.Tissue Eng Part B Rev. 2022 Feb;28(1):160-181. doi: 10.1089/ten.TEB.2020.0339. Epub 2021 Feb 22. Tissue Eng Part B Rev. 2022. PMID: 33446047 Review.
Cited by
-
Astragalus polysaccharide-containing 3D-printed scaffold for traumatized skin repair and proteomic study.J Cell Mol Med. 2024 Aug;28(16):e70023. doi: 10.1111/jcmm.70023. J Cell Mol Med. 2024. PMID: 39158533 Free PMC article.
-
Genetic and bioactive functionalization of bioinks for 3D bioprinting.Bioprocess Biosyst Eng. 2025 Sep;48(9):1421-1449. doi: 10.1007/s00449-025-03180-y. Epub 2025 May 20. Bioprocess Biosyst Eng. 2025. PMID: 40392297 Review.
-
Stimulation by exosomes from hypoxia-preconditioned hair follicle mesenchymal stem cells facilitates mitophagy by inhibiting the PI3K/AKT/mTOR signaling pathway to alleviate ulcerative colitis.Theranostics. 2024 Jul 8;14(11):4278-4296. doi: 10.7150/thno.96038. eCollection 2024. Theranostics. 2024. PMID: 39113800 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

