Evaluating potential predictors of weight loss response to liraglutide in adolescents with obesity: A post hoc analysis of the randomized, placebo-controlled SCALE Teens trial
- PMID: 37264767
- PMCID: PMC10926323
- DOI: 10.1111/ijpo.13061
Evaluating potential predictors of weight loss response to liraglutide in adolescents with obesity: A post hoc analysis of the randomized, placebo-controlled SCALE Teens trial
Abstract
Background: As childhood obesity prevalence increases, determining which patients respond to anti-obesity medications would strengthen personalized approaches to obesity treatment. In the SCALE Teens trial among pubertal adolescents with obesity (NCT02918279), liraglutide 3.0 mg (or maximum tolerated dose) significantly reduced body mass index (BMI) standard deviation score on average versus placebo. That said, liraglutide effects on BMI reduction varied greatly among adolescents, similar to adults.
Objectives: To identify post hoc characteristics predictive of achieving ≥5% and ≥10% BMI reductions at 56 weeks with liraglutide versus placebo in adolescents from the SCALE Teens trial.
Methods: Logistic regression analysis was performed in 251 adolescents treated with liraglutide (n = 125) or placebo (n = 126) for 56 weeks. Baseline characteristics (selected a priori) included sex, race, ethnicity, age, Tanner (pubertal) stage, glycemic status (hyperglycemia [type 2 diabetes/prediabetes] vs. normoglycemia), obesity category (Class II/III vs. I), severity of depression symptoms (Patient Health Questionnaire-9), and weight variability (weight fluctuations over time). The effects of early responder status (≥4% BMI reduction at week 16) on week 56 response were assessed using descriptive statistics.
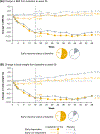
Results: Baseline characteristics did not affect achievement of ≥5% and ≥10% BMI reductions at week 56 in adolescents treated with liraglutide. Further, there was no association between weight variability and BMI reduction. Early liraglutide responders appeared to have greater BMI and body weight reductions at week 56 compared with early non-responders.
Conclusions: This secondary analysis suggests that adolescents with obesity may experience significant BMI reductions after 56 weeks of liraglutide treatment, regardless of their sex, race, ethnicity, age, pubertal stage, glycemic status, obesity category, severity of depression symptoms, or weight variability. Early response may predict greater week 56 response.
Keywords: anti-agents; glucagon-like peptide-1 receptor agonists; liraglutide; obesity; paediatric obesity; weight management.
© 2023 The Authors. Pediatric Obesity published by John Wiley & Sons Ltd on behalf of World Obesity Federation.
Conflict of interest statement
Megan O. Bensignor received research support from Vivus Inc (donated study drug) and Abbott (donated Freestyle Libre Continuous Glucose Monitoring systems). Carolyn T. Bramante received payment or honoraria for the Columbia Obesity Medicine Course. Amy C. Gross reports no competing interests. Claudia K. Fox and Eric M. Bomberg report acting as site principal investigators and co-investigators for Novo Nordisk. Paula M. Hale is an employee and shareholder of Novo Nordisk and received support for attending meetings including travel. Nina M. Harder-Lauridsen is an employee and shareholder of Novo Nordisk. Nandana Prabhu, and Rashmi K Mamadi are both employees of Novo Nordisk. Aaron S. Kelly engages in unpaid consulting and educational activities for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus; receives donated drug/placebo from Novo Nordisk and Vivus for National Institute of Diabetes and Digestive and Kidney Diseases-funded clinical trials.
Figures


References
-
- World Health Organization. Obesity and overweight. Accessed February 11, 2022. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
-
- Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity as a disease: the Obesity Society 2018 position statement. Obesity (Silver Spring). 2019;27(1):7–9. - PubMed
-
- Thomas-Eapen N. Childhood obesity. Prim Care. 2021;48(3):505–515. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

