An Updated Pooled Analysis of Off-Label Under and Over-Dosed Direct Oral Anticoagulants in Patients with Atrial Fibrillation
- PMID: 37264798
- PMCID: PMC10272681
- DOI: 10.1177/10760296231179439
An Updated Pooled Analysis of Off-Label Under and Over-Dosed Direct Oral Anticoagulants in Patients with Atrial Fibrillation
Abstract
Introduction: Off-label, under-, and overdosed direct oral anticoagulants (DOACs) are commonly prescribed to patients with atrial fibrillation (AF), but real-world evidence on their effectiveness and safety is limited.
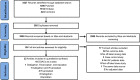
Methods: MEDLINE, Embase, and Cochrane Library databases were systematically searched from 01 July 2020 to 28 February 2022 to update a previous systematic review with the same search strategy from the inception to 30 June 2020. Eligible studies were those that reported effectiveness (stroke/systemic embolism and myocardial infarction) or safety (gastrointestinal or major bleeding and death) outcomes of off-label doses of DOACs compared to on-label doses in AF patients. A random-effects meta-analysis was performed to estimate the pooled hazard ratio (HR) and 95% confidence interval (CI). Subgroup analyses were performed by specific DOACs and geographic regions.
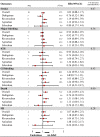
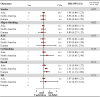
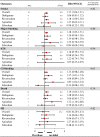
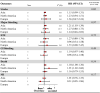
Results: Twenty-two studies were included. Off-label, underdosed DOACs, compared to on-label doses, were not associated with an increased risk of stroke (HR 1.03, 95%CI: 0.88-1.17) but were associated with an increased risk of death (HR 1.26, 95%CI: 1.09-1.43). However, risk varied depending on the active ingredient. No other safety outcomes were associated with underdosed DOACs. No significant differences were observed by geographic regions. Compared to on-label DOACs, overdosing increased the risk of stroke (HR 1.17, 95%CI: 1.04-1.31), major bleeding (HR 1.18, 95%CI: 1.05-1.31), and death (HR 1.19, 95%CI: 1.03-1.35). Risk varied between geographical regions.
Conclusions: Off-label underdoses, compared to on-label dosing of DOACs, did not increase the risk of stroke but did increase overall mortality. Overdosed DOACs, compared to on-label doses, were associated with an increased risk of stroke, major bleeding, and death. Future studies must examine these associations, focusing on specific active ingredients and geographic settings.
Keywords: atrial fibrillation; bleeding; direct oral anticoagulants; off-label; stroke.
Figures
References
-
- Atrial Fibrillation - Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/atrial_fibrillation.htm#:∼:text=Atrial%...
-
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. - PubMed
-
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. - PubMed
-
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. - PubMed
-
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

