Deficiency in hereditary hemorrhagic telangiectasia-associated Endoglin elicits hypoxia-driven heart failure in zebrafish
- PMID: 37264878
- PMCID: PMC10245139
- DOI: 10.1242/dmm.049488
Deficiency in hereditary hemorrhagic telangiectasia-associated Endoglin elicits hypoxia-driven heart failure in zebrafish
Abstract
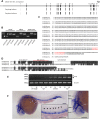
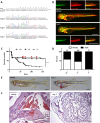
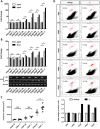
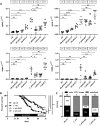
Hereditary hemorrhagic telangiectasia (HHT) is a rare genetic disease caused by mutations affecting components of bone morphogenetic protein (BMP)/transforming growth factor-β (TGF-β) signaling in endothelial cells. This disorder is characterized by arteriovenous malformations that are prone to rupture, and the ensuing hemorrhages are responsible for iron-deficiency anemia. Along with activin receptor-like kinase (ALK1), mutations in endoglin are associated with the vast majority of HHT cases. In this study, we characterized the zebrafish endoglin locus and demonstrated that it produces two phylogenetically conserved protein isoforms. Functional analysis of a CRISPR/Cas9 zebrafish endoglin mutant revealed that Endoglin deficiency is lethal during the course from juvenile stage to adulthood. Endoglin-deficient zebrafish develop cardiomegaly, resulting in heart failure and hypochromic anemia, which both stem from chronic hypoxia. endoglin mutant zebrafish display structural alterations of the developing gills and underlying vascular network that coincide with hypoxia. Finally, phenylhydrazine treatment demonstrated that lowering hematocrit/blood viscosity alleviates heart failure and enhances the survival of Endoglin-deficient fish. Overall, our data link Endoglin deficiency to heart failure and establish zebrafish as a valuable HHT model.
Keywords: Cardiomegaly; Endoglin; Endothelial cells; HHT; Heart failure; Hypoxia.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
-
- Baeyens, N., Larrivée, B., Ola, R., Hayward-Piatkowskyi, B., Dubrac, A., Huang, B., Ross, T. D., Coon, B. G., Min, E., Tsarfati, M.et al. (2016). Defective fluid shear stress mechanotransduction mediates hereditary hemorrhagic telangiectasia. J. Cell Biol. 214, 807-816. 10.1083/jcb.201603106 - DOI - PMC - PubMed
-
- Bellón, T., Corbí, A., Lastres, P., Calés, C., Cebrián, M., Vera, S., Cheifetz, S., Massague, J., Letarte, M. and Bernabéu, C. (1993). Identification and expression of two forms of the human transforming growth factor-beta-binding protein endoglin with distinct cytoplasmic regions. Eur. J. Immunol. 23, 2340-2345. 10.1002/eji.1830230943 - DOI - PubMed
-
- Blanco, F. J., Grande, M. T., Langa, C., Oujo, B., Velasco, S., Rodriguez-Barbero, A., Perez-Gomez, E., Quintanilla, M., López-Novoa, J. M. and Bernabeu, C. (2008). S-endoglin expression is induced in senescent endothelial cells and contributes to vascular pathology. Circ. Res. 103, 1383-1392. 10.1161/CIRCRESAHA.108.176552 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

