SARS-CoV-2 ORF8 Mediates Signals in Macrophages and Monocytes through MyD88 Independently of the IL-17 Receptor
- PMID: 37265402
- PMCID: PMC10330444
- DOI: 10.4049/jimmunol.2300110
SARS-CoV-2 ORF8 Mediates Signals in Macrophages and Monocytes through MyD88 Independently of the IL-17 Receptor
Abstract
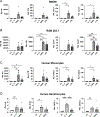
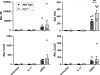
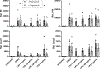
SARS-CoV-2 has caused an estimated 7 million deaths worldwide to date. A secreted SARS-CoV-2 accessory protein, known as open reading frame 8 (ORF8), elicits inflammatory pulmonary cytokine responses and is associated with disease severity in COVID-19 patients. Recent reports proposed that ORF8 mediates downstream signals in macrophages and monocytes through the IL-17 receptor complex (IL-17RA, IL-17RC). However, generally IL-17 signals are found to be restricted to the nonhematopoietic compartment, thought to be due to rate-limiting expression of IL-17RC. Accordingly, we revisited the capacity of IL-17 and ORF8 to induce cytokine gene expression in mouse and human macrophages and monocytes. In SARS-CoV-2-infected human and mouse lungs, IL17RC mRNA was undetectable in monocyte/macrophage populations. In cultured mouse and human monocytes and macrophages, ORF8 but not IL-17 led to elevated expression of target cytokines. ORF8-induced signaling was fully preserved in the presence of anti-IL-17RA/RC neutralizing Abs and in Il17ra-/- cells. ORF8 signaling was also operative in Il1r1-/- bone marrow-derived macrophages. However, the TLR/IL-1R family adaptor MyD88, which is dispensable for IL-17R signaling, was required for ORF8 activity yet MyD88 is not required for IL-17 signaling. Thus, we conclude that ORF8 transduces inflammatory signaling in monocytes and macrophages via MyD88 independently of the IL-17R.
Copyright © 2023 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors declare no conflicts of interest
Figures



References
-
- Moore JB, and June CH 2020. Cytokine release syndrome in severe COVID-19. Science 368: 473–474. - PubMed
-
- Young BE, Fong SW, Chan YH, Mak TM, Ang LW, Anderson DE, Lee CY, Amrun SN, Lee B, Goh YS, Su YCF, Wei WE, Kalimuddin S, Chai LYA, Pada S, Tan SY, Sun L, Parthasarathy P, Chen YYC, Barkham T, Lin RTP, Maurer-Stroh S, Leo YS, Wang LF, Renia L, Lee VJ, Smith GJD, Lye DC, and Ng LFP 2020. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet 396: 603–611. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

