Preclinical Characterization and Phase I Trial Results of INBRX-109, A Third-Generation, Recombinant, Humanized, Death Receptor 5 Agonist Antibody, in Chondrosarcoma
- PMID: 37265425
- PMCID: PMC10425732
- DOI: 10.1158/1078-0432.CCR-23-0974
Preclinical Characterization and Phase I Trial Results of INBRX-109, A Third-Generation, Recombinant, Humanized, Death Receptor 5 Agonist Antibody, in Chondrosarcoma
Abstract
Purpose: Patients with unresectable/metastatic chondrosarcoma have poor prognoses; conventional chondrosarcoma is associated with a median progression-free survival (PFS) of <4 months after first-line chemotherapy. No standard targeted therapies are available. We present the preclinical characterization of INBRX-109, a third-generation death receptor 5 (DR5) agonist, and clinical findings from a phase I trial of INBRX-109 in unresectable/metastatic chondrosarcoma (NCT03715933).
Patients and methods: INBRX-109 was first characterized preclinically as a DR5 agonist, with binding specificity and hepatotoxicity evaluated in vitro and antitumor activity evaluated both in vitro and in vivo. INBRX-109 (3 mg/kg every 3 weeks) was then evaluated in a phase I study of solid tumors, which included a cohort with any subtype of chondrosarcoma and a cohort with IDH1/IDH2-mutant conventional chondrosarcoma. The primary endpoint was safety. Efficacy was an exploratory endpoint, with measures including objective response, disease control rate, and PFS.
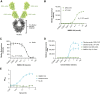
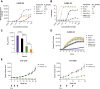
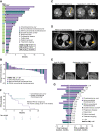
Results: In preclinical studies, INBRX-109 led to antitumor activity in vitro and in patient-derived xenograft models, with minimal hepatotoxicity. In the phase I study, INBRX-109 was well tolerated and demonstrated antitumor activity in unresectable/metastatic chondrosarcoma. INBRX-109 led to a disease control rate of 87.1% [27/31; durable clinical benefit, 40.7% (11/27)], including two partial responses, and median PFS of 7.6 months. Most treatment-related adverse events, including liver-related events, were low grade (grade ≥3 events in chondrosarcoma cohorts, 5.7%).
Conclusions: INBRX-109 demonstrated encouraging antitumor activity with a favorable safety profile in patients with unresectable/metastatic chondrosarcoma. A randomized, placebo-controlled, phase II trial (ChonDRAgon, NCT04950075) will further evaluate INBRX-109 in conventional chondrosarcoma.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- WHO Classification of Tumours editorial board, editor. WHO classification of tumours. Soft tissue and bone tumours. 5 ed. Volume3. Lyon: IARC Press; 2020.
-
- Unni KK, Inwards CY. Dahlin's bone tumors: general aspects and data on 10,165 cases. Philadelphia: Wollters Kluwer Health/Lippincott Williams & Wilkins; 2010, vii. p.402.
-
- Picci P, Manfrini M, Donati DM, Gambarotti M, Righi A, Vanel D, et al. Diagnosis of musculoskeletal tumors and tumor-like conditions: clinical, radiological and histological correlations-the Rizzoli case archive. Springer; 2020.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

