Type 2 inflammation and biological therapies in asthma: Targeted medicine taking flight
- PMID: 37265457
- PMCID: PMC10239209
- DOI: 10.1084/jem.20221212
Type 2 inflammation and biological therapies in asthma: Targeted medicine taking flight
Abstract
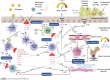
The field of asthma has undergone a dramatic change in recent years. Advances in our understanding of type 2 airway inflammation have driven the discovery of monoclonal antibodies targeting specific aspects of the immune pathway. In landmark trials, these drugs have shown efficacy in reducing asthma attacks and exposure to oral corticosteroids, important causes of morbidity in people with asthma. Our review explores the key features of type 2 inflammation in asthma and summarizes the clinical trial evidence of the novel monoclonal antibody treatments and future avenues for treatment.
© 2023 Howell et al.
Conflict of interest statement
Disclosures: I.D. Pavord reported having, over the last 5 years, received speaker’s honoraria for speaking at sponsored meetings from Astra Zeneca, Boehringer Inglehiem, Aerocrine, Almirall, Novartis, Teva, Chiesi, Sanofi/Regeneron, Menarini, and GSK, as well as payments for organizing educational events from Astra Zeneca, GSK, Sanofi/Regeneron, and Teva. He has also received honoraria for attending advisory panels with Genentech, Sanofi/Regeneron, Astra Zeneca, Boehringer Ingelheim, GSK, Novartis, Teva, Merck, Circassia, Chiesi, and Knopp, as well as payments to support FDA approval meetings from GSK; receiving sponsorship to attend international scientific meetings from Boehringer Ingelheim, GSK, Astra Zeneca, Teva, and Chiesi; and has received a grant from Chiesi to support a phase 2 clinical trial in Oxford. No other disclosures were reported.
Figures
References
-
- Altman, M.C., Gill M.A., Whalen E., Babineau D.C., Shao B., Liu A.H., Jepson B., Gruchalla R.S., O’Connor G.T., Pongracic J.A., et al. . 2019. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat. Immunol. 20:637–651. 10.1038/s41590-019-0347-8 - DOI - PMC - PubMed
-
- Bachert, C., Han J.K., Desrosiers M., Hellings P.W., Amin N., Lee S.E., Mullol J., Greos L.S., Bosso J.V., Laidlaw T.M., et al. . 2019. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 394:1638–1650. 10.1016/S0140-6736(19)31881-1 - DOI - PubMed

