Pulmonary bacteriophage and cystic fibrosis airway mucus: friends or foes?
- PMID: 37265479
- PMCID: PMC10230084
- DOI: 10.3389/fmed.2023.1088494
Pulmonary bacteriophage and cystic fibrosis airway mucus: friends or foes?
Abstract
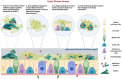
For those born with cystic fibrosis (CF), hyper-concentrated mucus with a dysfunctional structure significantly impacts CF airways, providing a perfect environment for bacterial colonization and subsequent chronic infection. Early treatment with antibiotics limits the prevalence of bacterial pathogens but permanently alters the CF airway microenvironment, resulting in antibiotic resistance and other long-term consequences. With little investment into new traditional antibiotics, safe and effective alternative therapeutic options are urgently needed. One gathering significant traction is bacteriophage (phage) therapy. However, little is known about which phages are effective for respiratory infections, the dynamics involved between phage(s) and the host airway, and associated by-products, including mucus. Work utilizing gut cell models suggest that phages adhere to mucus components, reducing microbial colonization and providing non-host-derived immune protection. Thus, phages retained in the CF mucus layer result from the positive selection that enables them to remain in the mucus layer. Phages bind weakly to mucus components, slowing down the diffusion motion and increasing their chance of encountering bacterial species for subsequent infection. Adherence of phage to mucus could also facilitate phage enrichment and persistence within the microenvironment, resulting in a potent phage phenotype or vice versa. However, how the CF microenvironment responds to phage and impacts phage functionality remains unknown. This review discusses CF associated lung diseases, the impact of CF mucus, and chronic bacterial infection. It then discusses the therapeutic potential of phages, their dynamic relationship with mucus and whether this may enhance or hinder airway bacterial infections in CF.
Keywords: airway epithelium; antimicrobial resistance; bacteriophage; cystic fibrosis; mucus.
Copyright © 2023 Ling, Stick and Kicic.
Conflict of interest statement
The authors declare that the review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- O’Neil J. (2014). Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Available at: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-act... [Accessed August 31, 2021]
-
- Anitha Kopinathan, Halili Maria A., Begona Heras, Scanlon Martin J., Makrina Totsika, JL Martin AC. (2021). The next pandemic: antimicrobial resistance - Curious. Available at: https://www.science.org.au/curious/policy-features/next-pandemic-antimic... [Accessed November 23, 2021]
-
- Chan BK, Abedon ST. Phage therapy pharmacology. Phage cocktails In Laskin Allen I, Sariaslani Sima, Gadd Geoffrey M, editors. Advances in applied microbiology. 78: Taylor & Francis; (2012). 1–23. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

