The role of infected epithelial cells in Chlamydia-associated fibrosis
- PMID: 37265500
- PMCID: PMC10230099
- DOI: 10.3389/fcimb.2023.1208302
The role of infected epithelial cells in Chlamydia-associated fibrosis
Abstract
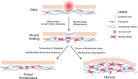
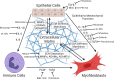
Ocular, genital, and anogenital infection by the obligate intracellular pathogen Chlamydia trachomatis have been consistently associated with scar-forming sequelae. In cases of chronic or repeated infection of the female genital tract, infection-associated fibrosis of the fallopian tubes can result in ectopic pregnancy or infertility. In light of this urgent concern to public health, the underlying mechanism of C. trachomatis-associated scarring is a topic of ongoing study. Fibrosis is understood to be an outcome of persistent injury and/or dysregulated wound healing, in which an aberrantly activated myofibroblast population mediates hypertrophic remodeling of the basement membrane via deposition of collagens and other components of the extracellular matrix, as well as induction of epithelial cell proliferation via growth factor signaling. Initial study of infection-associated immune cell recruitment and pro-inflammatory signaling have suggested the cellular paradigm of chlamydial pathogenesis, wherein inflammation-associated tissue damage and fibrosis are the indirect result of an immune response to the pathogen initiated by host epithelial cells. However, recent work has revealed more direct routes by which C. trachomatis may induce scarring, such as infection-associated induction of growth factor signaling and pro-fibrotic remodeling of the extracellular matrix. Additionally, C. trachomatis infection has been shown to induce an epithelial-to-mesenchymal transition in host epithelial cells, prompting transdifferentiation into a myofibroblast-like phenotype. In this review, we summarize the field's current understanding of Chlamydia-associated fibrosis, reviewing key new findings and identifying opportunities for further research.
Keywords: Chlamydia trachomatis; ECM; EMT; fibrosis; host-pathogen interaction; pathogenesis.
Copyright © 2023 Caven and Carabeo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Chlamydial YAP activation in host endocervical epithelial cells mediates pro-fibrotic paracrine stimulation of fibroblasts.bioRxiv [Preprint]. 2023 May 31:2023.05.30.542940. doi: 10.1101/2023.05.30.542940. bioRxiv. 2023. Update in: mSystems. 2023 Dec 21;8(6):e0090423. doi: 10.1128/msystems.00904-23. PMID: 37398163 Free PMC article. Updated. Preprint.
-
Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring.Infect Immun. 1997 Jun;65(6):2175-82. doi: 10.1128/iai.65.6.2175-2182.1997. Infect Immun. 1997. PMID: 9169748 Free PMC article.
-
Chlamydial YAP activation in host endocervical epithelial cells mediates pro-fibrotic paracrine stimulation of fibroblasts.mSystems. 2023 Dec 21;8(6):e0090423. doi: 10.1128/msystems.00904-23. Epub 2023 Oct 24. mSystems. 2023. PMID: 37874141 Free PMC article.
-
Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections.Contraception. 2015 Aug;92(2):108-15. doi: 10.1016/j.contraception.2015.01.004. Epub 2015 Jan 13. Contraception. 2015. PMID: 25592078 Review.
-
Chlamydia trachomatis: the Persistent Pathogen.Clin Vaccine Immunol. 2017 Oct 5;24(10):e00203-17. doi: 10.1128/CVI.00203-17. Print 2017 Oct. Clin Vaccine Immunol. 2017. PMID: 28835360 Free PMC article. Review.
Cited by
-
Morphology of the immune cells in the wall of the human uterine tube and their possible impact on reproduction-uterine tube as a possible immune privileged organ.Front Cell Dev Biol. 2024 Mar 7;12:1325565. doi: 10.3389/fcell.2024.1325565. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38516130 Free PMC article. Review.
-
A novel multi-variate immunological approach, reveals immune variation associated with environmental conditions, and co-infection in the koala (Phascolarctos cinereus).Sci Rep. 2024 Mar 27;14(1):7260. doi: 10.1038/s41598-024-57792-7. Sci Rep. 2024. PMID: 38538683 Free PMC article.
-
Serum IgG1 and IgG3 Antibody Responses to Chlamydia trachomatis Pgp3 and Hsp60 in Tubal Factor Infertility.J Infect Dis. 2025 Jul 11;231(6):e1057-e1064. doi: 10.1093/infdis/jiaf092. J Infect Dis. 2025. PMID: 39981649 Free PMC article.
-
Role of microRNAs in immune regulation and pathogenesis of Chlamydia trachomatis and Chlamydia muridarum infections: a rapid review.Microbes Infect. 2024 Nov-Dec;26(8):105397. doi: 10.1016/j.micinf.2024.105397. Epub 2024 Jul 17. Microbes Infect. 2024. PMID: 39025257 Review.
-
Endoplasmic reticulum: the target of chlamydial manipulation.Arch Microbiol. 2025 Aug 11;207(9):219. doi: 10.1007/s00203-025-04411-2. Arch Microbiol. 2025. PMID: 40788340 Review.
References
-
- Adami E., Viswanathan S., Widjaja A. A., Ng B., Chothani S., Zhihao N., et al. . (2021). IL11 is elevated in systemic sclerosis and IL11-dependent ERK signalling underlies TGFβ-mediated activation of dermal fibroblasts. Rheumatology 60, 5820–5826. doi: 10.1093/rheumatology/keab168 - DOI - PMC - PubMed
-
- Akcora B. Ö., Storm G., Bansal R. (2018). Inhibition of canonical WNT signaling pathway by β-catenin/CBP inhibitor ICG-001 ameliorates liver fibrosis in vivo through suppression of stromal CXCL12. Biochim. Biophys. Acta Mol. Basis. Dis. 1864, 804–818. doi: 10.1016/j.bbadis.2017.12.001 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

