Pretreatment Vitamin D Concentrations Do Not Predict Therapeutic Outcome to Anti-TNF Therapies in Biologic-Naïve Patients With Active Luminal Crohn's Disease
- PMID: 37265586
- PMCID: PMC10231451
- DOI: 10.1093/crocol/otad026
Pretreatment Vitamin D Concentrations Do Not Predict Therapeutic Outcome to Anti-TNF Therapies in Biologic-Naïve Patients With Active Luminal Crohn's Disease
Abstract
Background and aims: Vitamin D has a regulatory role in innate and adaptive immune processes. Previous studies have reported that low pretreatment vitamin D concentrations are associated with primary non-response (PNR) and non-remission to anti-TNF therapy. This study aimed to assess whether pretreatment 25-hydroxyvitamin D concentrations predicted PNR and non-remission to infliximab and adalimumab in patients with active luminal Crohn's disease.
Methods: 25-Hydroxyvitamin D concentrations were measured in stored baseline samples from 659 infliximab- and 448 adalimumab-treated patients in the Personalised Anti-TNF Therapy in Crohn's disease (PANTS) study. Cut-offs for vitamin D were deficiency <25 nmol/L, insufficiency 25-50 nmol/L, and adequacy/sufficiency >50 nmol/L.
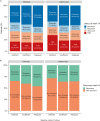
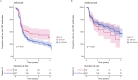
Results: About 17.1% (189/1107; 95% CI, 15.0-19.4) and 47.7% (528/1107; 95% CI, 44.8-50.6) of patients had vitamin D deficiency and insufficiency, respectively. 22.2% (246/1107) of patients were receiving vitamin D supplementation. Multivariable analysis confirmed that sampling during non-summer months, South Asian ethnicity, lower serum albumin concentrations, and non-treatment with vitamin D supplementation were independently associated with lower vitamin D concentrations. Pretreatment vitamin D status did not predict response or remission to anti-TNF therapy at week 14 (infliximab Ppnr = .89, adalimumab Ppnr = .18) or non-remission at week 54 (infliximab P = .13, adalimumab P = .58). Vitamin D deficiency was, however, associated with a longer time to immunogenicity in patients treated with infliximab, but not adalimumab.
Conclusions: Vitamin D deficiency is common in patients with active Crohn's disease. Unlike previous studies, pretreatment vitamin D concentration did not predict PNR to anti-TNF treatment at week 14 or nonremission at week 54.
Keywords: Crohn’s disease; IBD; PANTS; vitamin D.
© The Author(s) 2023. Published by Oxford University Press on behalf of Crohn's & Colitis Foundation.
Conflict of interest statement
S.L. reports nonfinancial support from Pfizer outside the submitted work. N.A.K. reports grants from F. Hoffmann-La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, nonfinancial support from Immundiagnostik, grants and nonfinancial support from AbbVie, grants and personal fees from Celltrion, personal fees and nonfinancial support from Janssen, personal fees from Takeda, personal fees and nonfinancial support from Dr Falk, outside the submitted work. T.A. reports grants and nonfinancial support from F. Hoffmann-La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, nonfinancial support from Immundiagnostik, personal fees from Biogen inc, grants and personal fees from Celltrion Healthcare, personal fees and nonfinancial support from Immundiagnostik, personal fees from Takeda, personal fees from ARENA, personal fees from Gilead, personal fees from Adcock Ingram Healthcare, personal fees from Pfizer, personal fees from Genentech, nonfinancial support from Tillotts, outside the submitted work. J.R.G. reports grants from F. Hoffmann-La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, nonfinancial support from Immundiagnostik, outside the submitted work. The following authors have nothing to declare: N.C., R.S., C.R., R.N., T.J.M., C.B., B.H., and M.B.
Figures


Similar articles
-
Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study.Lancet Gastroenterol Hepatol. 2019 May;4(5):341-353. doi: 10.1016/S2468-1253(19)30012-3. Epub 2019 Feb 27. Lancet Gastroenterol Hepatol. 2019. PMID: 30824404
-
Mechanisms and management of loss of response to anti-TNF therapy for patients with Crohn's disease: 3-year data from the prospective, multicentre PANTS cohort study.Lancet Gastroenterol Hepatol. 2024 Jun;9(6):521-538. doi: 10.1016/S2468-1253(24)00044-X. Epub 2024 Apr 16. Lancet Gastroenterol Hepatol. 2024. PMID: 38640937
-
Understanding anti-TNF treatment failure: does serum triiodothyronine-to-thyroxine (T3/T4) ratio predict therapeutic outcome to anti-TNF therapies in biologic-naïve patients with active luminal Crohn's disease?Aliment Pharmacol Ther. 2022 Sep;56(5):783-793. doi: 10.1111/apt.17089. Epub 2022 Jun 29. Aliment Pharmacol Ther. 2022. PMID: 35768996 Free PMC article.
-
[Anti-TNF therapy in treatment of luminal Crohn's disease].Acta Med Croatica. 2013 Apr;67(2):179-89. Acta Med Croatica. 2013. PMID: 24471301 Review. Croatian.
-
Management of inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies.BioDrugs. 2010 Dec 14;24 Suppl 1:3-14. doi: 10.2165/11586290-000000000-00000. BioDrugs. 2010. PMID: 21175228 Review.
Cited by
-
Correlation between Trabecular Bone Score and Homocysteine Level in Rheumatoid Arthritis Patients on Anti-TNF Inhibitors.Life (Basel). 2024 Apr 1;14(4):463. doi: 10.3390/life14040463. Life (Basel). 2024. PMID: 38672734 Free PMC article.
-
Evaluating the predictive effect of vitamin D on clinical outcomes of infliximab-treated Crohn's disease patients in western China.Clin Exp Med. 2024 Oct 4;24(1):237. doi: 10.1007/s10238-024-01483-0. Clin Exp Med. 2024. PMID: 39365401 Free PMC article.
-
The Role of Vitamin D in Inflammatory Bowel Diseases: From Deficiency to Targeted Therapeutics and Precise Nutrition Strategies.Nutrients. 2025 Jun 29;17(13):2167. doi: 10.3390/nu17132167. Nutrients. 2025. PMID: 40647273 Free PMC article. Review.
-
Cellular and Molecular Determinants of Biologic Drugs Resistance and Therapeutic Failure in Inflammatory Bowel Disease.Int J Mol Sci. 2024 Feb 28;25(5):2789. doi: 10.3390/ijms25052789. Int J Mol Sci. 2024. PMID: 38474034 Free PMC article. Review.
-
Nutritional Deficiencies and Reduced Bone Mineralization in Ulcerative Colitis.J Clin Med. 2025 May 6;14(9):3202. doi: 10.3390/jcm14093202. J Clin Med. 2025. PMID: 40364233 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
