Improving osteoinduction and osteogenesis of Ti6Al4V alloy porous scaffold by regulating the pore structure
- PMID: 37265590
- PMCID: PMC10229796
- DOI: 10.3389/fchem.2023.1190630
Improving osteoinduction and osteogenesis of Ti6Al4V alloy porous scaffold by regulating the pore structure
Abstract
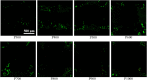
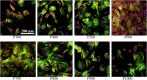
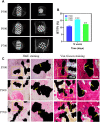
Titanium alloy scaffolds with a porous structure have attracted much attention in bone defect repair. However, which pore structure is more beneficial to bone defect repair is controversial. In the present research, the Ti6Al4V alloy porous scaffolds with gradient pore sizes were designed and fabricated. The microstructure characterization, tests of mechanical properties, and in vitro and in vivo experiments have been performed to systematically evaluate the effect of pore size on osteoinduction and osteogenesis. The results revealed that the contact angle with water, compressive strength, and elastic modulus of the Ti6Al4V alloy porous scaffolds decreased gradually with the increase of pore size. However, there were obvious drops when the pore size of the porous scaffold was around 600 μm. As the pore size increased, the proliferation and integrin β1 of RAW 264.7 macrophages seeded on Ti6Al4V alloy porous scaffolds increased at first, reaching a maximum value at a pore size of around 600 μm, and then decreased subsequently. The proliferation, integrin β1, and osteogenic gene-related expressions of Bone marrow mesenchymal stem cells (BMSCs) seeded on Ti6Al4V alloy porous scaffolds with different pore sizes all exhibited similar variations which rose with increased pore size firstly, obtaining the maximum value at pore size about 600 μm, and then declined. The in vivo experiments confirmed the in vitro results, and the Ti6Al4V alloy porous scaffold with a pore size of 600 μm possessed the better capability to induce new bone formation. Therefore, for the design of Ti6Al4V alloy with a regular porous scaffold, the surface morphology, porosity, strength, and elastic modulus should be considered systematically, which would determine the capability of osteoinduction and osteogenesis.
Keywords: Ti6Al4V alloy; bone defect repairing; osteogenesis; pore structure; porous scaffold.
Copyright © 2023 Wang, Wu, Liu, Xu, Liu, Li, Hou, Wang, Chen, Sheng, Lin and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Large-pore-size Ti6Al4V scaffolds with different pore structures for vascularized bone regeneration.Mater Sci Eng C Mater Biol Appl. 2021 Dec;131:112499. doi: 10.1016/j.msec.2021.112499. Epub 2021 Oct 19. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 34857285
-
Influence of the pore size and porosity of selective laser melted Ti6Al4V ELI porous scaffold on cell proliferation, osteogenesis and bone ingrowth.Mater Sci Eng C Mater Biol Appl. 2020 Jan;106:110289. doi: 10.1016/j.msec.2019.110289. Epub 2019 Oct 7. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31753386
-
Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes.J Mech Behav Biomed Mater. 2018 Aug;84:1-11. doi: 10.1016/j.jmbbm.2018.04.010. Epub 2018 Apr 18. J Mech Behav Biomed Mater. 2018. PMID: 29709846
-
3D-printed porous Ti6Al4V scaffolds for long bone repair in animal models: a systematic review.J Orthop Surg Res. 2022 Feb 2;17(1):68. doi: 10.1186/s13018-022-02960-6. J Orthop Surg Res. 2022. PMID: 35109907 Free PMC article.
-
Biomechanics of Additively Manufactured Metallic Scaffolds-A Review.Materials (Basel). 2021 Nov 12;14(22):6833. doi: 10.3390/ma14226833. Materials (Basel). 2021. PMID: 34832234 Free PMC article. Review.
Cited by
-
3D-printed porous titanium suture anchor: a rabbit lateral femoral condyle model.BMC Musculoskelet Disord. 2024 Jul 18;25(1):559. doi: 10.1186/s12891-024-07666-w. BMC Musculoskelet Disord. 2024. PMID: 39026178 Free PMC article.
-
Effect of Printing Parameters on the Dynamic Characteristics of Additively Manufactured ABS Beams: An Experimental Modal Analysis and Response Surface Methodology.Polymers (Basel). 2025 Jun 10;17(12):1615. doi: 10.3390/polym17121615. Polymers (Basel). 2025. PMID: 40574143 Free PMC article.
-
3D-Printed Polycaprolactone/Hydroxyapatite Bionic Scaffold for Bone Regeneration.Polymers (Basel). 2025 Mar 23;17(7):858. doi: 10.3390/polym17070858. Polymers (Basel). 2025. PMID: 40219249 Free PMC article.
-
3D printing of different fibres towards HA/PCL scaffolding induces macrophage polarization and promotes osteogenic differentiation of BMSCs.PLoS One. 2025 Jan 13;20(1):e0314150. doi: 10.1371/journal.pone.0314150. eCollection 2025. PLoS One. 2025. PMID: 39804888 Free PMC article.
-
The rational design, biofunctionalization and biological properties of orthopedic porous titanium implants: a review.Front Bioeng Biotechnol. 2025 Feb 26;13:1548675. doi: 10.3389/fbioe.2025.1548675. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40078794 Free PMC article. Review.
References
-
- Barth H. D., Zimmermann E. A., Schaible E., Tang S. Y., Alliston T., Ritchie R. O. (2011). Characterization of the effects of x-ray irradiation on the hierarchical structure and mechanical properties of human cortical bone. Biomaterials 32 (34), 8892–8904. 10.1016/j.biomaterials.2011.08.013 - DOI - PMC - PubMed
-
- Berry D. J., Harmsen W. S., Cabanela M. E., Morrey B. F. (2002). Twenty-five-year survivorship of two thousand consecutive primary charnley total hip replacements: Factors affecting survivorship of acetabular and femoral components. J. Bone Jt. Surg. Am. 84 (2), 171–177. 10.2106/00004623-200202000-00002 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

