Thermo-induced physically crosslinked polypeptide-based block copolymer hydrogels for biomedical applications
- PMID: 37265604
- PMCID: PMC10229375
- DOI: 10.1093/rb/rbad039
Thermo-induced physically crosslinked polypeptide-based block copolymer hydrogels for biomedical applications
Abstract
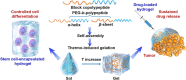
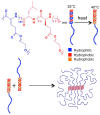
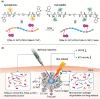
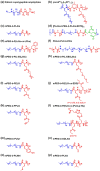
Stimuli-responsive synthetic polypeptide-containing block copolymers have received considerable attention in recent years. Especially, unique thermo-induced sol-gel phase transitions were observed for elaborately-designed amphiphilic diblock copolypeptides and a range of poly(ethylene glycol) (PEG)-polypeptide block copolymers. The thermo-induced gelation mechanisms involve the evolution of secondary conformation, enhanced intramolecular interactions, as well as reduced hydration and increased chain entanglement of PEG blocks. The physical parameters, including polymer concentrations, sol-gel transition temperatures and storage moduli, were investigated. The polypeptide hydrogels exhibited good biocompatibility in vitro and in vivo, and displayed biodegradation periods ranging from 1 to 5 weeks. The unique thermo-induced sol-gel phase transitions offer the feasibility of minimal-invasive injection of the precursor aqueous solutions into body, followed by in situ hydrogel formation driven by physiological temperature. These advantages make polypeptide hydrogels interesting candidates for diverse biomedical applications, especially as injectable scaffolds for 3D cell culture and tissue regeneration as well as depots for local drug delivery. This review focuses on recent advances in the design and preparation of injectable, thermo-induced physically crosslinked polypeptide hydrogels. The influence of composition, secondary structure and chirality of polypeptide segments on the physical properties and biodegradation of the hydrogels are emphasized. Moreover, the studies on biomedical applications of the hydrogels are intensively discussed. Finally, the major challenges in the further development of polypeptide hydrogels for practical applications are proposed.
Keywords: immunotherapy; injectable hydrogels; polypeptides; secondary conformation; stimuli responsive; tissue engineering.
© The Author(s) 2023. Published by Oxford University Press.
Figures











References
-
- He C, Zhuang X, Tang Z, Tian H, Chen X.. Stimuli-sensitive synthetic polypeptide-based materials for drug and gene delivery. Adv Healthc Mater 2012;1:48–78. - PubMed
-
- Deng C, Wu J, Cheng R, Meng F, Klok H-A, Zhong Z.. Functional polypeptide and hybrid materials: precision synthesis via alpha-amino acid N-carboxyanhydride polymerization and emerging biomedical applications. Prog Polym Sci 2014;39:330–64.
-
- He X, Fan JW, Wooley KL.. Stimuli-Triggered Sol–Gel transitions of polypeptides derived from -alpha-Amino acid N-Carboxyanhydride (NCA) polymerizations. Chem Asian J 2016;11:437–47. - PubMed
-
- Deming TJ. Cobalt and iron initiators for the controlled polymerization of α-amino acid-N-carboxyanhydrides. Macromolecules 1999;32:4500–2.
-
- Deming TJ. Facile synthesis of block copolypeptides of defined architecture. Nature 1997;390:386–9. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

