Copper-catalyzed [1,3]-nitrogen rearrangement of O- aryl ketoximes via oxidative addition of N-O bond in inverse electron flow
- PMID: 37265725
- PMCID: PMC10231427
- DOI: 10.1039/d3sc00874f
Copper-catalyzed [1,3]-nitrogen rearrangement of O- aryl ketoximes via oxidative addition of N-O bond in inverse electron flow
Abstract
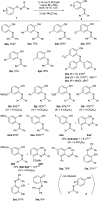
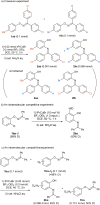
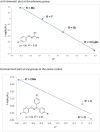
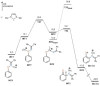
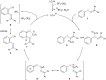
The [1,3]-nitrogen rearrangement reactions of O-aryl ketoximes were promoted by N-heterocyclic carbene (NHC)-copper catalysts and BF3·OEt2 as an additive, affording ortho-aminophenol derivatives in good yields. The reaction of substrates with electron-withdrawing substituents on the phenol moiety are accelerated by adding silver salt and modifying the substituent at the nitrogen atom. Density functional theory calculations suggest that the rate-determining step of this reaction is the oxidative addition of the N-O bond of the substrate to the copper catalyst. The negative ρ values of the substituent at both the oxime carbon and phenoxy group indicate that the donation of electrons by the oxygen and nitrogen atoms accelerates the oxidative addition.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Narasaka K. Kitamura M. Eur. J. Org. Chem. 2005:4505–4519. doi: 10.1002/ejoc.200500389. - DOI
- Patureau F. W. Glorius F. Angew. Chem., Int. Ed. 2011;50:1977–1979. doi: 10.1002/anie.201007241. - DOI - PubMed
- Huang H. Ji X. Wu W. Jiang H. Chem. Soc. Rev. 2015;44:1155–1171. doi: 10.1039/C4CS00288A. - DOI - PubMed
- Huang H. Cai J. Deng G.-J. Org. Biomol. Chem. 2016;14:1519–1530. doi: 10.1039/C5OB02417J. - DOI - PubMed
- Rykaczewski K. A. Wearing E. R. Blackmun D. E. Schindler C. S. Nat. Synth. 2022;1:24–36. doi: 10.1038/s44160-021-00007-y. - DOI
- Hirano K. Miura M. J. Am. Chem. Soc. 2022;144:648–661. doi: 10.1021/jacs.1c12663. - DOI - PubMed
-
- Yang H.-B. Pathipati S. R. Selander N. ACS Catal. 2017;7:8441–8445. doi: 10.1021/acscatal.7b03432. - DOI
- Yu X.-Y. Zhao Q.-Q. Chen J. Chen J.-R. Xiao W.-J. Angew. Chem., Int. Ed. 2018;57:15505–15509. doi: 10.1002/anie.201809820. - DOI - PubMed
- Nakahuku K. M. Zhang Z. Wappes E. A. Stateman L. M. Chen A. D. Nagib D. A. Nat. Chem. 2020;12:697–704. doi: 10.1038/s41557-020-0482-8. - DOI - PMC - PubMed
- Becker M. R. Wearing E. R. Schindler C. S. Nat. Chem. 2020;12:898–905. doi: 10.1038/s41557-020-0541-1. - DOI - PubMed
- Du F. Jiang S.-J. Zeng R. Pan X.-C. Lan Y. Chen Y.-C. Wei Y. Angew. Chem., Int. Ed. 2020;59:23755–23762. doi: 10.1002/anie.202010752. - DOI - PubMed
- Górski B. Barthelemy A.-L. Douglas J. J. Juliá F. Leonori D. Nat. Catal. 2021;4:54–61. doi: 10.1038/s41929-020-00553-2. - DOI
- Wei W.-X. Li Y. Wen Y.-T. Li M. Li X.-S. Wang C.-T. Liu H.-C. Xia Y. Zhang B.-S. Jiao R.-Q. Liang Y.-M. J. Am. Chem. Soc. 2021;143:7868–7875. doi: 10.1021/jacs.1c04114. - DOI - PubMed
- Wearing E. R. Blackmun D. E. Becker M. R. Schindler C. S. J. Am. Chem. Soc. 2021;143:16235–16242. doi: 10.1021/jacs.1c07523. - DOI - PubMed
- Fazekas T. J. Alty J. W. Neidhart E. K. Miller A. S. Leibfarth F. A. Alexanian E. J. Science. 2022;375:545–550. doi: 10.1126/science.abh4308. - DOI - PMC - PubMed
- Feng T. Liu C. Wu Z. Wu X. Zhu C. Chem. Sci. 2022;13:2669–2673. doi: 10.1039/D2SC00015F. - DOI - PMC - PubMed
-
- Hayashi H. Uchida T. Eur. J. Org. Chem. 2020:909–916. doi: 10.1002/ejoc.201901562. - DOI
-
, and references therein;
- Wang H. Jung H. Song F. Zhu S. Bai Z. Chen D. He G. Chang S. Chen G. Nat. Chem. 2021;13:378–385. doi: 10.1038/s41557-021-00650-0. - DOI - PubMed
- Lee E. Hwang Y. Kim Y. B. Kim D. Chang S. J. Am. Chem. Soc. 2021;143:6363–6369. doi: 10.1021/jacs.1c02550. - DOI - PubMed
- Du B. Ouyang Y. Chen Q. Yu W.-Y. J. Am. Chem. Soc. 2021;143:14962–14968. doi: 10.1021/jacs.1c05834. - DOI - PubMed
- Zhang Y. Qiao D. Duan M. Wang Y. Zhu S. Nat. Commun. 2022;13:5630. doi: 10.1038/s41467-022-33411-9. - DOI - PMC - PubMed
- Carlberg N. W. Rovis T. J. Am. Chem. Soc. 2022;144:22426–22432. doi: 10.1021/jacs.2c10552. - DOI - PMC - PubMed
- Li N. Zhao H. J. Gu Z. Angew. Chem., Int. Ed. 2023;62:e202215530. - PubMed
-
- Kikugawa Y. Heterocycles. 2009;78:571–607. doi: 10.3987/REV-08-644. - DOI
-
, and references therein;
- Nakamura I. Jo T. Tashiro H. Terada M. Org. Lett. 2017;19:3059–3062. doi: 10.1021/acs.orglett.7b01110. - DOI - PubMed
- Farndon J. J. Ma X. Bower J. F. J. Am. Chem. Soc. 2017;139:14005. doi: 10.1021/jacs.7b07830. - DOI - PMC - PubMed
- Ishida Y. Nakamura I. Terada M. J. Am. Chem. Soc. 2018;140:8629–8633. doi: 10.1021/jacs.8b03669. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

