Radical ring-opening polymerization of sustainably-derived thionoisochromanone
- PMID: 37265728
- PMCID: PMC10231309
- DOI: 10.1039/d2sc06040j
Radical ring-opening polymerization of sustainably-derived thionoisochromanone
Erratum in
-
Correction: Radical ring-opening polymerization of sustainably-derived thionoisochromanone.Chem Sci. 2023 Jun 12;14(24):6806. doi: 10.1039/d3sc90098c. eCollection 2023 Jun 21. Chem Sci. 2023. PMID: 37350818 Free PMC article.
Abstract
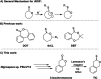
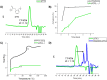
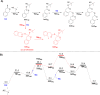
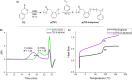
We present the synthesis, characterization and radical ring-opening polymerization (rROP) capabilities of thionoisochromanone (TIC), a fungi-derivable thionolactone. TIC is the first reported six-membered thionolactone to readily homopolymerize under free radical conditions without the presence of a dormant comonomer or repeated initiation. Even more, the resulting polymer is fully degradable under mild, basic conditions. Computations providing molecular-level insights into the mechanistic and energetic details of polymerization identified a unique S,S,O-orthoester intermediate that leads to a sustained chain-end. This sustained chain-end allowed for the synthesis of a block copolymer of TIC and styrene under entirely free radical conditions without explicit radical control methods such as reversible addition-fragmentation chain transfer polymerization (RAFT). We also report the statistical copolymerization of ring-retained TIC and styrene, confirmed by elemental analysis and energy-dispersive X-ray spectroscopy (EDX). Computations into the energetic details of copolymerization indicate kinetic drivers for ring-retaining behavior. This work provides the first example of a sustainable feedstock for rROP and provides the field with the first six-membered monomer susceptible to rROP, expanding the monomer scope to aid our fundamental understanding of thionolactone rROP behavior.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Öztürk T. Savaş B. Meyvacı E. Kılıçlıoğlu A. Hazer B. Synthesis and Characterization of the Block Copolymers Using the Novel Bifunctional Initiator by RAFT and FRP Technics: Evaluation of the Primary Polymerization Parameters. J. Polym. Res. 2020;27(3):1–7. doi: 10.1007/s10965-020-2043-7. - DOI
-
- Barner-kowollik C. Buback M. Charleux B. Coote M. L. Drache M. Fukuda T. Goto A. Klumperman B. Lowe A. B. Mcleary J. B. Moad G. Monteiro M. J. Sanderson R. D. Tonge M. P. Vana P. Mechanism and Kinetics of Dithiobenzoate-Mediated RAFT Polymerization. I. The Current Situation. J. Polym. Sci., Part A: Polym. Chem. 2006;44:5809–5831. doi: 10.1002/pola. doi: 10.1002/pola.21589. - DOI - DOI
LinkOut - more resources
Full Text Sources

