Randomized evaluation of routine beta-blocker therapy after myocardial infarction quality of life (RQoL): design and rationale of a multicentre, prospective, randomized, open, blinded endpoint study
- PMID: 37265820
- PMCID: PMC10230287
- DOI: 10.1093/ehjopen/oead036
Randomized evaluation of routine beta-blocker therapy after myocardial infarction quality of life (RQoL): design and rationale of a multicentre, prospective, randomized, open, blinded endpoint study
Abstract
Aims: Most cases of acute myocardial infarction (MI) in Sweden are treated with long-term β-blocker therapy as secondary prevention. Case studies and patient reports have indicated negative effects of β-blockers including symptoms of depression, fatigue, sexual dysfunction, and general low mood, all related to reduced quality of life (QoL). To date, no recent large-scale, randomized trial has explored the effects of β-blockers on these factors.
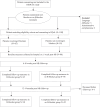
Methods and results: The ongoing Randomized Evaluation of Decreased Usage of beta-bloCkErs after myocardial infarction (REDUCE): quality of life (RQoL) study is a multicentre, prospective, randomized pre-specified substudy aiming to evaluate the effects of β-blockers on self-reported measures of QoL. Following randomized allocation to long-term β-blocker or no β-blocker treatment, patients complete a total of six baseline measures pertaining to QoL, sexual functioning, and perceived side effects. Data collection is optionally carried out online through a unique and secure portal and repeated again at two follow-up time points. Recruitment began in July 2018. Data from the first 100 patients showed that at the first follow-up, 93% had completed the questionnaires, which decreased to 81% at the second follow-up. The method of digital data collection was utilized by over half of the patients recruited so far.
Conclusion: Data from the first 100 patients indicate success in terms of study design and recruitment. The RQoL substudy investigates the effects of β-blockers on self-reported measures of QoL in MI patients and will potentially contribute to the limited knowledge of QoL-related side effects reported in conjunction with β-blocker use.
Clinical trial registration: Eudra CT number, 2017-002336-17; Clinical trial.gov identifier, NCT03278509.
Keywords: Adrenergic beta-antagonist; Digital data collection; Myocardial infarction; Nationwide register data; Quality of life; Randomized clinical trial.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: No relationship with industry or conflict of interest relevant for this trial were reported.
Figures
References
-
- Hjalmarson Å, Herlitz J, Málek I, Rydén L, Vedin A, Waldenström A, Wedel H, Elmfeldt D, Holmberg S, Nyberg G, Swedberg K, Waagstein F, Waldenström J, Wilhelmsen L, Wilhelmsson C. Effect on mortality of metoprolol in acute myocardial infarction. A double-blind randomised trial. Lancet 1981;318:823–827. - PubMed
-
- National Heart, Lung, and Blood Institute . A randomized trial of propranolol in patients with acute myocardial infarction: I. Mortality results. JAMA 1982;247:1707–1714. - PubMed
-
- Metoprolol in acute myocardial infarction (MIAMI). A randomised placebo-controlled international trial. The MIAMI Trial Research Group. Eur Heart J 1985;6:199–226. - PubMed
-
- Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group . 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2020;74:544.
Associated data
LinkOut - more resources
Full Text Sources
Medical