Botryosphaerialean fungi associated with woody oil plants cultivated in Sichuan Province, China
- PMID: 37265995
- PMCID: PMC10230375
- DOI: 10.3897/mycokeys.97.103118
Botryosphaerialean fungi associated with woody oil plants cultivated in Sichuan Province, China
Abstract
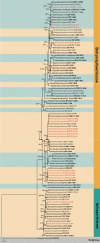
Woody oil plants are important economic trees which are widely cultivated and distributed throughout China. Surveys conducted during 2020 and 2021 on several woody oil plantations from five regions of Sichuan Province, China, revealed a high diversity of Botryosphaerialean fungi. The identification of 50 botryosphaeriaceous isolates was carried out based on both morphology and multi-gene phylogenetic analysis of internal transcribed spacer region (ITS), translation elongation factor 1-alpha gene (tef1) and β-tubulin gene (tub2). This allowed the identification of twelve previously known Botryosphaeriales species: Aplosporellaprunicola, A.ginkgonis, Barriopsistectonae, Botryosphaeriadothidea, Bo.fabicerciana, Diplodiamutila, Di.seriata, Dothiorellasarmentorum, Neofusicoccumparvum, Sardiniellaguizhouensis, Sphaeropsiscitrigena, and Sp.guizhouensis, and four novel species belonging to the genera Diplodia and Dothiorella, viz. Di.acerigena, Di.pistaciicola, Do.camelliae and Do.zanthoxyli. The dominant species isolated across the surveyed regions were Botryosphaeriadothidea, Sardiniellaguizhouensis and Diplodiamutila, representing 20%, 14% and 12% of the total isolates, respectively. In addition, most isolates were obtained from Pistaciachinensis (14 isolates), followed by Camelliaoleifera (10 isolates). The present study enhances the understanding of Botryosphaeriales species diversity on woody oil plants in Sichuan Province, China.
Keywords: Botryosphaeriales; diversity; new species; phylogeny; taxonomy.
Wen-Li Li, Rui-Ru Liang, Asha J. Dissanayake, Jian-Kui Liu.
Figures


















References
-
- Batista E, Lopes A, Alves A. (2021) What do we know about Botryosphaeriaceae? An overview of a Worldwide Cured Dataset. Forests 12(3): e313. 10.3390/f12030313 - DOI
-
- Carbone I, Kohn LM. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91(3): 553–556. 10.1080/00275514.1999.12061051 - DOI
LinkOut - more resources
Full Text Sources
