Cytokine signaling converging on IL11 in ILD fibroblasts provokes aberrant epithelial differentiation signatures
- PMID: 37266432
- PMCID: PMC10230276
- DOI: 10.3389/fimmu.2023.1128239
Cytokine signaling converging on IL11 in ILD fibroblasts provokes aberrant epithelial differentiation signatures
Abstract
Introduction: Interstitial lung disease (ILD) is a heterogenous group of lung disorders where destruction and incomplete regeneration of the lung parenchyma often results in persistent architectural distortion of the pulmonary scaffold. Continuous mesenchyme-centered, disease-relevant signaling likely initiates and perpetuates the fibrotic remodeling process, specifically targeting the epithelial cell compartment, thereby destroying the gas exchange area.
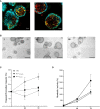
Methods: With the aim of identifying functional mediators of the lung mesenchymal-epithelial crosstalk with potential as new targets for therapeutic strategies, we developed a 3D organoid co-culture model based on human induced pluripotent stem cell-derived alveolar epithelial type 2 cells that form alveolar organoids in presence of lung fibroblasts from fibrotic-ILD patients, in our study referring to cases of pulmonary fibrosis, as well as control cell line (IMR-90).
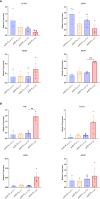
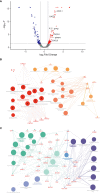
Results: While organoid formation capacity and size was comparable in the presence of fibrotic-ILD or control lung fibroblasts, metabolic activity was significantly increased in fibrotic-ILD co-cultures. Alveolar organoids cultured with fibrotic-ILD fibroblasts further demonstrated reduced stem cell function as reflected by reduced Surfactant Protein C gene expression together with an aberrant basaloid-prone differentiation program indicated by elevated Cadherin 2, Bone Morphogenic Protein 4 and Vimentin transcription. To screen for key mediators of the misguided mesenchymal-to-epithelial crosstalk with a focus on disease-relevant inflammatory processes, we used mass spectrometry and characterized the secretome of end stage fibrotic-ILD lung fibroblasts in comparison to non-chronic lung disease (CLD) patient fibroblasts. Out of the over 2000 proteins detected by this experimental approach, 47 proteins were differentially abundant comparing fibrotic-ILD and non-CLD fibroblast secretome. The fibrotic-ILD secretome profile was dominated by chemokines, including CXCL1, CXCL3, and CXCL8, interfering with growth factor signaling orchestrated by Interleukin 11 (IL11), steering fibrogenic cell-cell communication, and proteins regulating extracellular matrix remodeling including epithelial-to-mesenchymal transition. When in turn treating alveolar organoids with IL11, we recapitulated the co-culture results obtained with primary fibrotic-ILD fibroblasts including changes in metabolic activity.
Conclusion: We identified mediators likely contributing to the disease-perpetuating mesenchymal-to-epithelial crosstalk in ILD. In our alveolar organoid co-cultures, we were able to highlight the importance of fibroblast-initiated aberrant epithelial differentiation and confirmed IL11 as a key player in fibrotic-ILD pathogenesis by unbiased fibroblast secretome analysis.
Keywords: IL11; co-culture model; cytokine; disease modeling; human pluripotent stem cells; interstitial lung disease; organoids; secretome.
Copyright © 2023 Kastlmeier, Gonzalez-Rodriguez, Cabanis, Guenther, König, Han, Hauck, See, Asgharpour, Bukas, Burgstaller, Piraud, Lehmann, Hatz, Behr, Stoeger, Hilgendorff and Voss.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

