HIV-1 genotypic profiling ensures effective response to third-line antiretroviral therapy in Cameroon
- PMID: 37266631
- PMCID: PMC10238024
- DOI: 10.1097/MD.0000000000033897
HIV-1 genotypic profiling ensures effective response to third-line antiretroviral therapy in Cameroon
Abstract
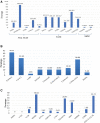
In order to limit the emergence of human immunodeficiency virus (HIV) drug resistance in a context of limited antiretroviral options, we sought to evaluate the efficacy of third-line (3L) regimens considering HIV genotypic resistance profile at initiation of 3L in Cameroon. A cohort-study was conducted from January-September 2020 among patients initiating a 3L antiretroviral therapy regimen at the Yaoundé Central Hospital. HIV-1 protease-reverse transcriptase was sequenced at the Chantal Biya international reference center for research on HIV/AIDS prevention and management and results were interpreted using Stanford HIVdbv8.3. Good virological response (viral load < 390 copies/mL) was assessed after 12 months using OPP-ERA platform. Statistical analyses were performed using Epi Info v7.2.2.6, with P < .05 considered statistically significant. Of the 38 patients initiating 3L with an available genotyping (42% female; median age, 49 [39-57] years), median cluster of differentiation type 4 count and viral load were 173 [34-374] cells/μL and 169,322 [30,382-551,826] copies/mL, respectively. At enrollment, all patients harbored resistance to reverse transcriptase inhibitors and 66% (25/38) to protease-inhibitors, although 63% (24/38) were still susceptible to darunavir/ritonavir. Preferred 3L regimen was dolutegravir + darunavir/r + tenofovir + lamivudine (51%) and median duration on 3L was 21 [17-32] months. Interestingly, 82% (31/38) of the participants achieved good virological response on 3L, regardless of genotypic profile at recruitment, variations in 3L regimens (P = .9) and baseline cluster of differentiation type 4 count (P = .3). Despite the high burden of reverse transcriptase inhibitor - and protease inhibitor boosted by ritonavir drug resistance, genotyping-guided 3L regimens is accompanied by virological success in most patients. This high efficacy, most likely due to use of high genetic barrier antiretrovirals, requires continuous adherence support alongside close monitoring for long-term effectiveness in similar programmatic settings.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
References
-
- ONUSIDA. Fiche d’information – Dernières statistiques sur l’état de l’épidémie de sida | ONUSIDA. 2022. Available at: https://www.unaids.org/fr/resources/fact-sheet. [Access date April 13, 2022].
-
- Ekollo Mbange A, Malick Diouara AA, Diop-Ndiaye H, et al. . High HIV-1 virological failure and drug resistance among adult patients receiving first-line ART for at least 12 months at a decentralized urban HIV clinic setting in Senegal before the test-and-treat. Infect Dis (Auckl). 2021;14:11786337211014504. - PMC - PubMed
-
- WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2016. Available at: https://www.who.int/publications/i/item/9789241549684. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

