Effectiveness of a Quality Improvement Intervention on Reperfusion Treatment for Patients With Acute Ischemic Stroke: A Stepped-Wedge Cluster Randomized Clinical Trial
- PMID: 37266940
- PMCID: PMC10238948
- DOI: 10.1001/jamanetworkopen.2023.16465
Effectiveness of a Quality Improvement Intervention on Reperfusion Treatment for Patients With Acute Ischemic Stroke: A Stepped-Wedge Cluster Randomized Clinical Trial
Abstract
Importance: Reperfusion therapy is the most effective treatment for acute ischemic stroke but remains underused in China.
Objective: To evaluate the effect of a problem-oriented, culturally adapted, targeted quality improvement intervention on reperfusion therapy for patients with acute ischemic stroke in China.
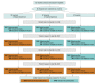
Design, setting, and participants: In this stepped-wedge cluster randomized clinical trial, patients from 16 secondary and 33 tertiary hospitals in China with acute ischemic stroke within 6 hours of symptom onset were consecutively recruited between July 1, 2018, and June 30, 2020.
Interventions: Hospitals were randomly assigned to 1 of 3 sequences to receive the targeted quality improvement intervention (n = 5689), in which workflow reconstruction was promoted to reduce in-hospital reperfusion treatment delays, or usual care (n = 6443), in which conventional stroke care was left to the discretion of the stroke team.
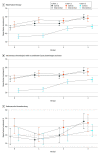
Main outcomes and measures: The primary outcome was the reperfusion therapy rate, a composite outcome of intravenous recombinant tissue plasminogen activator (IV rtPA) or endovascular thrombectomy (EVT) for eligible patients who arrived within 3.5 or 4.5 hours of symptom onset. Secondary outcomes were the IV rtPA administration rate among eligible patients who arrived within 3.5 hours of symptom onset, the EVT rate among eligible participants who arrived within 4.5 hours of symptom onset, the proportion of patients with door-to-needle time within 60 minutes, the proportion of patients with door-to-puncture time within 90 minutes, in-hospital mortality, and 3-month disability as measured by a modified Rankin Scale score greater than 2.
Results: All 12 132 eligible patients (mean [SD] age, 66 [12.1] years; 7759 male [64.0%]) completed the trial. The reperfusion rate was 53.5% (3046 of 5689) for the eligible patients in the intervention period and 43.9% (2830 of 6443) in the control period. No significant improvement in primary outcomes was found for the intervention after adjusting for cluster, period, and imbalanced baseline covariates (adjusted risk difference [ARD], 5.5%; 95% CI, -8.0% to 19.0%; adjusted odds ratio [AOR], 1.26; 95% CI, 0.72-2.21) or for the secondary outcomes. However, significant improvements were found in secondary hospitals for reperfusion therapy (1081 of 1870 patients [57.8%] vs 945 of 2022 patients [42.9%]; ARD, 19.0%; 95% CI, 6.4%-31.6%; AOR, 2.24; 95% CI, 1.29-3.88), IV rtPA administration (1062 of 1826 patients [58.2%] vs 916 of 2170 patients [42.2%]; ARD, 20.3%; 95% CI, 7.4%-33.1%; AOR, 2.37; 95% CI, 1.34-4.19), and EVT (51 of 231 patients [22.1%] vs 37 of 259 patients [14.3%]; ARD, 13.6%; 95% CI, 1.0%-26.3%; AOR, 3.03; 95% CI, 1.11-8.25) in subgroup analyses.
Conclusions and relevance: In this stepped-wedge cluster randomized clinical trial of patients with acute ischemic stroke in China, the use of a targeted quality improvement intervention compared with usual care did not improve the reperfusion therapy rate. However, the intervention may be effective in secondary hospitals.
Trial registration: ClinicalTrials.gov Identifier: NCT03578107.
Conflict of interest statement
Figures

References
-
- Vos T, Lim SS, Abbafati C, et al. ; GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. doi:10.1016/S0140-6736(20)30925-9 - DOI - PMC - PubMed
-
- Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi:10.1016/S0140-6736(14)60584-5 - DOI - PMC - PubMed

