Direct Oral Anticoagulants vs Low-Molecular-Weight Heparin and Recurrent VTE in Patients With Cancer: A Randomized Clinical Trial
- PMID: 37266947
- PMCID: PMC10265290
- DOI: 10.1001/jama.2023.7843
Direct Oral Anticoagulants vs Low-Molecular-Weight Heparin and Recurrent VTE in Patients With Cancer: A Randomized Clinical Trial
Abstract
Importance: In patients with cancer who have venous thromboembolism (VTE) events, long-term anticoagulation with low-molecular-weight heparin (LMWH) is recommended to prevent recurrent VTE. The effectiveness of a direct oral anticoagulant (DOAC) compared with LMWH for preventing recurrent VTE in patients with cancer is uncertain.
Objective: To evaluate DOACs, compared with LMWH, for preventing recurrent VTE and for rates of bleeding in patients with cancer following an initial VTE event.
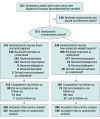
Design, setting, and participants: Unblinded, comparative effectiveness, noninferiority randomized clinical trial conducted at 67 oncology practices in the US that enrolled 671 patients with cancer (any invasive solid tumor, lymphoma, multiple myeloma, or chronic lymphocytic leukemia) who had a new clinical or radiological diagnosis of VTE. Enrollment occurred from December 2016 to April 2020. Final follow-up was in November 2020.
Intervention: Participants were randomized in a 1:1 ratio to either a DOAC (n = 335) or LMWH (n = 336) and were followed up for 6 months or until death. Physicians and patients selected any DOAC or any LMWH (or fondaparinux) and physicians selected drug doses.
Main outcomes and measures: The primary outcome was the recurrent VTE rate at 6 months. Noninferiority of anticoagulation with a DOAC vs LMWH was defined by the upper limit of the 1-sided 95% CI for the difference of a DOAC relative to LMWH of less than 3% in the randomized cohort that received at least 1 dose of assigned treatment. The 6 prespecified secondary outcomes included major bleeding, which was assessed using a 2.5% noninferiority margin.
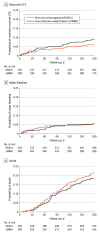
Results: Between December 2016 and April 2020, 671 participants were randomized and 638 (95%) completed the trial (median age, 64 years; 353 women [55%]). Among those randomized to a DOAC, 330 received at least 1 dose. Among those randomized to LMWH, 308 received at least 1 dose. Rates of recurrent VTE were 6.1% in the DOAC group and 8.8% in the LMWH group (difference, -2.7%; 1-sided 95% CI, -100% to 0.7%) consistent with the prespecified noninferiority criterion. Of 6 prespecified secondary outcomes, none were statistically significant. Major bleeding occurred in 5.2% of participants in the DOAC group and 5.6% in the LMWH group (difference, -0.4%; 1-sided 95% CI, -100% to 2.5%) and did not meet the noninferiority criterion. Severe adverse events occurred in 33.8% of participants in the DOAC group and 35.1% in the LMWH group. The most common serious adverse events were anemia and death.
Conclusions and relevance: Among adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE over 6-month follow-up. These findings support use of a DOAC to prevent recurrent VTE in patients with cancer.
Trial registration: ClinicalTrials.gov Identifier: NCT02744092.
Conflict of interest statement
Figures
Comment in
-
In adults with cancer and VTE, DOACs were noninferior to LMWH for recurrent, nonfatal VTE at 6 mo.Ann Intern Med. 2023 Sep;176(9):JC104. doi: 10.7326/J23-0067. Epub 2023 Sep 5. Ann Intern Med. 2023. PMID: 37665989
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

