Maternal PBDE exposure disrupts gut microbiome and promotes hepatic proinflammatory signaling in humanized PXR-transgenic mouse offspring over time
- PMID: 37267213
- PMCID: PMC10375318
- DOI: 10.1093/toxsci/kfad056
Maternal PBDE exposure disrupts gut microbiome and promotes hepatic proinflammatory signaling in humanized PXR-transgenic mouse offspring over time
Abstract
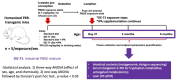
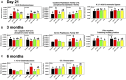
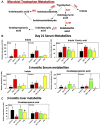
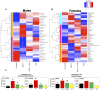
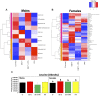
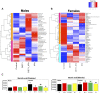
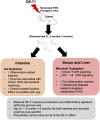
Developmental exposure to the persistent environmental pollutant, polybrominated diphenyl ethers (PBDEs), is associated with increased diabetes prevalence. The microbial tryptophan metabolite, indole-3-propionic acid (IPA), is associated with reduced risk of type 2 diabetes and lower-grade inflammation and is a pregnane X receptor (PXR) activator. To explore the role of IPA in modifying the PBDE developmental toxicity, we orally exposed humanized PXR-transgenic (hPXR-TG) mouse dams to vehicle, 0.1 mg/kg/day DE-71 (an industrial PBDE mixture), DE-71+IPA (20 mg/kg/day), or IPA, from 4 weeks preconception to the end of lactation. Pups were weaned at 21 days of age and IPA supplementation continued in the corresponding treatment groups. Tissues were collected at various ages until 6 months of age (n = 5 per group). In general, the effect of maternal DE-71 exposure on the gut microbiome of pups was amplified over time. The regulation of hepatic cytokines and prototypical xenobiotic-sensing transcription factor target genes by DE-71 and IPA was age- and sex-dependent, where DE-71-mediated mRNA increased selected cytokines (Il10, Il12p40, Il1β [both sexes], and [males]). The hepatic mRNA of the aryl hydrocarbon receptor (AhR) target gene Cyp1a2 was increased by maternal DE-71 and DE-71+IPA exposure at postnatal day 21 but intestinal Cyp1a1 was not altered by any of the exposures and ages. Maternal DE-71 exposure persistently increased serum indole, a known AhR ligand, in age- and sex-dependent manner. In conclusion, maternal DE-71 exposure produced a proinflammatory signature along the gut-liver axis, including gut dysbiosis, dysregulated tryptophan microbial metabolism, attenuated PXR signaling, and elevated AhR signaling in postweaned hPXR-TG pups over time, which was partially corrected by IPA supplementation.
Keywords: AhR; PBDE; PXR; diabetes; inflammation; metabolomics; microbiome.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Impacts of PBDE-47 exposure before, during and after pregnancy on the maternal gut microbiome and its association with host metabolism.Ecotoxicol Environ Saf. 2021 Oct 1;222:112530. doi: 10.1016/j.ecoenv.2021.112530. Epub 2021 Jul 17. Ecotoxicol Environ Saf. 2021. PMID: 34280840
-
Pregnane X receptor exacerbates nonalcoholic fatty liver disease accompanied by obesity- and inflammation-prone gut microbiome signature.Biochem Pharmacol. 2021 Nov;193:114698. doi: 10.1016/j.bcp.2021.114698. Epub 2021 Jul 23. Biochem Pharmacol. 2021. PMID: 34303710 Free PMC article.
-
Regulation of protein-coding gene and long noncoding RNA pairs in liver of conventional and germ-free mice following oral PBDE exposure.PLoS One. 2018 Aug 1;13(8):e0201387. doi: 10.1371/journal.pone.0201387. eCollection 2018. PLoS One. 2018. PMID: 30067809 Free PMC article.
-
Pregnane X Receptor and the Gut-Liver Axis: A Recent Update.Drug Metab Dispos. 2022 Apr;50(4):478-491. doi: 10.1124/dmd.121.000415. Epub 2021 Dec 3. Drug Metab Dispos. 2022. PMID: 34862253 Free PMC article. Review.
-
Activation of aryl hydrocarbon receptor (AhR) in Alzheimer's disease: role of tryptophan metabolites generated by gut host-microbiota.J Mol Med (Berl). 2023 Mar;101(3):201-222. doi: 10.1007/s00109-023-02289-5. Epub 2023 Feb 9. J Mol Med (Berl). 2023. PMID: 36757399 Free PMC article. Review.
Cited by
-
Multiomics-Based Profiling of the Fecal Microbiome Reveals Potential Disease-Specific Signatures in Pediatric IBD (PIBD).Biomolecules. 2025 May 21;15(5):746. doi: 10.3390/biom15050746. Biomolecules. 2025. PMID: 40427639 Free PMC article.
-
Exploratory Metabolomic and Lipidomic Profiling in a Manganese-Exposed Parkinsonism-Affected Population in Northern Italy.Metabolites. 2025 Jul 20;15(7):487. doi: 10.3390/metabo15070487. Metabolites. 2025. PMID: 40710587 Free PMC article.
-
Tryptophan in the mouse diet is essential for embryo implantation and decidualization.Front Endocrinol (Lausanne). 2024 May 1;15:1356914. doi: 10.3389/fendo.2024.1356914. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38752181 Free PMC article.
-
Single-cell transcriptomics showed that maternal PCB exposure dysregulated ER stress-mediated cell type-specific responses in the liver of female offspring.bioRxiv [Preprint]. 2025 Jun 8:2025.06.04.657944. doi: 10.1101/2025.06.04.657944. bioRxiv. 2025. PMID: 40501930 Free PMC article. Preprint.
-
Metabolomic and Lipidomic Analysis of Manganese-Associated Parkinsonism: a Case-Control Study in Brescia, Italy.medRxiv [Preprint]. 2024 Sep 6:2024.09.04.24313002. doi: 10.1101/2024.09.04.24313002. medRxiv. 2024. PMID: 39281765 Free PMC article. Preprint.
References
-
- Alamshah A., Spreckley E., Norton M., Kinsey-Jones J. S., Amin A., Ramgulam A., Cao Y., Johnson R., Saleh K., Akalestou E., et al. (2017). L-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int. J. Obes. (Lond.) 41, 1693–1701. - PMC - PubMed
-
- Azar N., Booij L., Muckle G., Arbuckle T. E., Séguin J. R., Asztalos E., Fraser W. D., Lanphear B. P., Bouchard M. F. (2021). Prenatal exposure to polybrominated diphenyl ethers (PBDEs) and cognitive ability in early childhood. Environ. Int. 146, 106296. - PubMed
-
- Bacanlı M., Anlar H. G., Aydın S., Çal T., Arı N., Ündeğer Bucurgat Ü., Başaran A. A., Başaran N. (2017). D-limonene ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 110, 434–442. - PubMed
-
- Bassols J., Serino M., Carreras-Badosa G., Burcelin R., Blasco-Baque V., Lopez-Bermejo A., Fernandez-Real J.-M. (2016). Gestational diabetes is associated with changes in placental microbiota and microbiome. Pediatr. Res. 80, 777–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

