Characteristics and prognostic impact of IDH mutations in AML: a COG, SWOG, and ECOG analysis
- PMID: 37267439
- PMCID: PMC10562769
- DOI: 10.1182/bloodadvances.2022008282
Characteristics and prognostic impact of IDH mutations in AML: a COG, SWOG, and ECOG analysis
Abstract
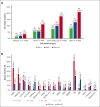
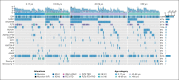
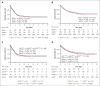
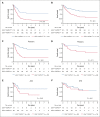
Somatic mutations in isocitrate dehydrogenase (IDH) genes occur frequently in adult acute myeloid leukemia (AML) and less commonly in pediatric AML. The objective of this study was to describe the prevalence, mutational profile, and prognostic significance of IDH mutations in AML across age. Our cohort included 3141 patients aged between <1 month and 88 years treated on Children's Cancer Group/Children's Oncology Group (n = 1872), Southwest Oncology Group (n = 359), Eastern Cooperative Oncology Group (n = 397) trials, and in Beat AML (n = 333) and The Cancer Genome Atlas (n = 180) genomic characterization cohorts. We retrospectively analyzed patients in 4 age groups (age range, n): pediatric (0-17, 1744), adolescent/young adult (18-39, 444), intermediate-age (40-59, 640), older (≥60, 309). IDH mutations (IDHmut) were identified in 9.2% of the total cohort (n = 288; IDH1 [n = 123, 42.7%]; IDH2 [n = 165, 57.3%]) and were strongly correlated with increased age: 3.4% pediatric vs 21% older, P < .001. Outcomes were similar in IDHmut and IDH-wildtype (IDHWT) AML (event-free survival [EFS]: 35.6% vs 40.0%, P = .368; overall survival [OS]: 50.3% vs 55.4%, P = .196). IDH mutations frequently occurred with NPM1 (47.2%), DNMT3A (29.3%), and FLT3-internal tandem duplication (ITD) (22.4%) mutations. Patients with IDHmut AML with NPM1 mutation (IDHmut/NPM1mut) had significantly improved survival compared with the poor outcomes experienced by patients without (IDHmut/NPM1WT) (EFS: 55.1% vs 17.0%, P < .001; OS: 66.5% vs 35.2%, P < .001). DNTM3A or FLT3-ITD mutations in otherwise favorable IDHmut/NPM1mut AML led to inferior outcomes. Age group analysis demonstrated that IDH mutations did not abrogate the favorable prognostic impact of NPM1mut in patients aged <60 years; older patients had poor outcomes regardless of NPM1 status. These trials were registered at www.clinicaltrials.gov as #NCT00070174, #NCT00372593, #NCT01371981, #NCT00049517, and #NCT00085709.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.O. reports consulting or an advisory role for GlycoMimetics, Cascadia Labs, Merck, Daiichi Sankyo, and BioSight. J.P.R. reports personal fees from Novartis, Bristol Myers Squibb, Takeda, Amgen, Cepheid, and Genentech outside the submitted work. M.S.T. reports grant/research support from AbbVie, Orsenix, Biosight, GlycoMimetics, Rafael Pharmaceuticals, and Amgen; a scientific advisory role for AbbVie, Orsenix, Biosight, Daiichi Sankyo Co., KAHR, Novartis Pharmaceuticals, and Innate Pharmaceuticals; and royalty from UpToDate. M.L. reports consulting or an advisory role for Omeros and Jazz Pharmaceuticals; reports research funding form Amgen, Astellas Pharma, Actinium Pharmaceuticals, Pluristem Therapeutics, AbbVie/Genentech, Tolero Pharmaceuticals, and AbbVie; and served on the data monitoring committee for BioSight. E.A. reports consultancy for AbbVie, Takeda, Pfizer, Novartis, and Amgen; speakers’ bureau role for AbbVie and Bristol Myers Squibb; received honoraria from Bristol Myers Squibb and Novartis; and received research funding from Takeda, Pfizer, and Novartis. O.A.-W. has served as a consultant for H3B Biomedicine, Foundation Medicine Inc., Merck, Prelude Therapeutics, and Janssen; is on the scientific advisory board of Envisagenics Inc., AIChemy, Harmonic Discovery Inc., and Pfizer Boulder; and has received prior research funding from H3B Biomedicine, Nurix Therapeutics, Minovia Therapeutics, and Loxo Oncology unrelated to the current manuscript. S.L. received honoraria from Syros, Agios, Daiichi Sankyo, Jazz Pharmaceuticals, Bristol Myers Squibb, Acceleron, Astellas, and Pfizer, and research funding from Onconova, Celgene, Biosight, Hoffman LaRoche, and Kura. H.E. reports consultancy or an advisory role for Agios, Astellas Pharma, Amgen, Celgene, Daiichi Sankyo, GlycoMimetics, Immunogen, Incyte, Jazz Pharmaceuticals, MacroGenics, Novartis, AbbVie/Genentech, Janssen Oncology, Pfizer, Trillium Therapeutics, Takeda, and Kura Oncology; a speakers’ bureau role for Agios, Celgene, Incyte, Jazz Pharmaceuticals, Novartis, and AbbVie/Genentech; received research funding from AbbVie, Agios (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Forma Therapeutics (Inst), Gilead/Forty Seven (Inst), Immunogen (Inst), Jazz Pharmaceuticals (Inst), MacroGenics (Inst), Novartis (Inst), PTC Therapeutics (Inst), AbbVie (Inst), GlycoMimetics (Inst), and ALX Oncology (Inst); declares other relationship with GlycoMimetics and Celgene; and reports an uncompensated relationship with Daiichi Sankyo. R.L. serves on the supervisory board of Qiagen; is a scientific adviser to Imago, Mission Bio, Syndax, Zentalis, Ajax, Bakx, Auron, Prelude, C4 Therapeutics, and Isoplexis for which he receives equity support; received research support from Ajax and AbbVie; reports consultancy for Incyte, Janssen, Morphosys, and Novartis; and received honoraria from AstraZeneca and Kura for invited lectures, and from Gilead for grant reviews. The remaining authors declare no competing financial interests.
Figures


References
-
- Meggendorfer M, Cappelli LV, Walter W, et al. IDH1R132, IDH2R140 and IDH2R172 in AML: different genetic landscapes correlate with outcome and may influence targeted treatment strategies. Leukemia. 2018;32(5):1249–1253. - PubMed
-
- Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28(22):3636–3643. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

