Treating Cancer in People With HIV
- PMID: 37267514
- PMCID: PMC10351946
- DOI: 10.1200/JCO.23.00737
Treating Cancer in People With HIV
Abstract
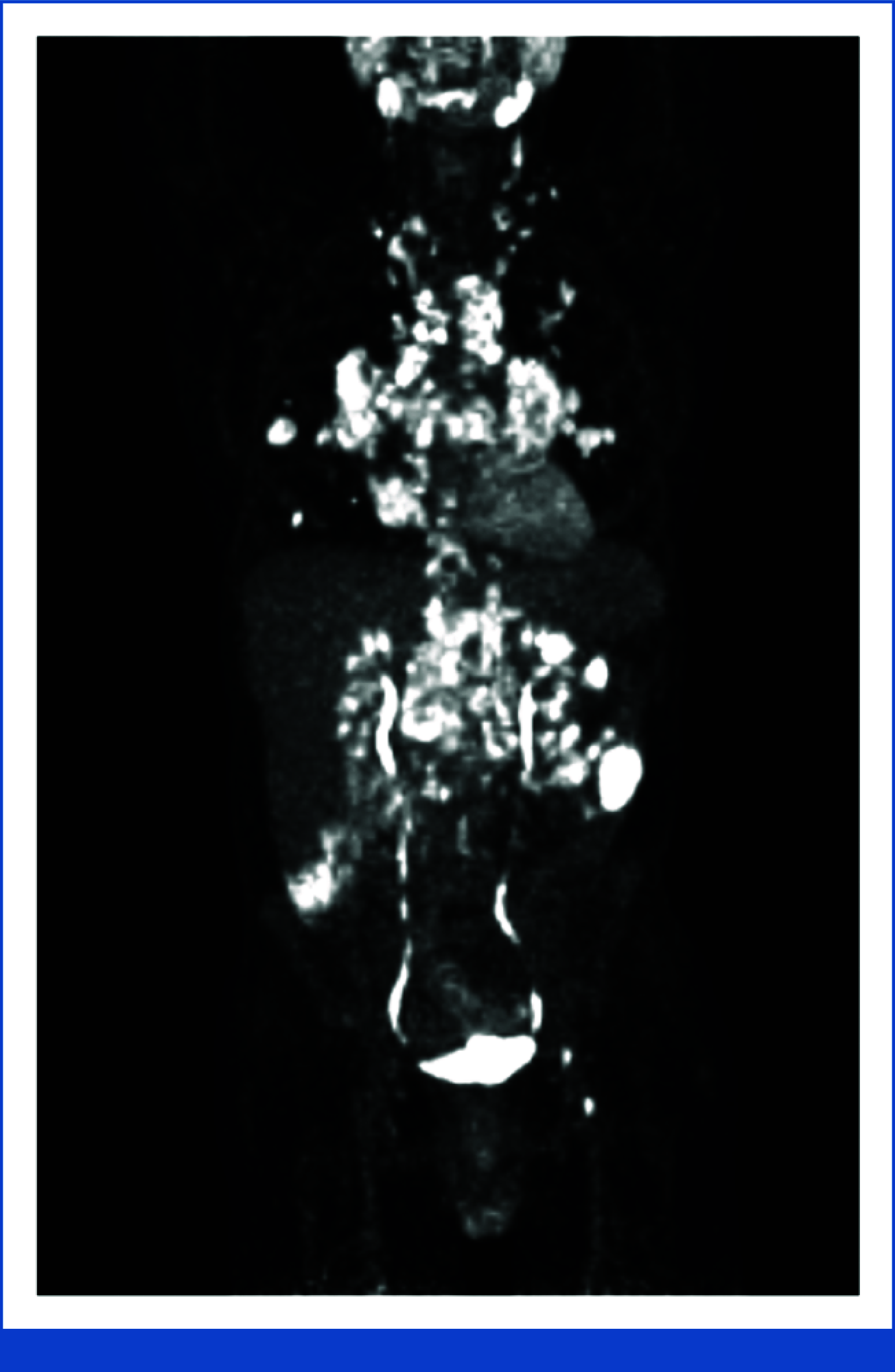
The Oncology Grand Rounds series is designed to place original reports published in the Journal into clinical context. A case presentation is followed by a description of diagnostic and management challenges, a review of the relevant literature, and a summary of the authors' suggested management approaches. The goal of this series is to help readers better understand how to apply the results of key studies, including those published in Journal of Clinical Oncology, to patients seen in their own clinical practice.People with HIV (PWH) have an increased lifetime risk of developing certain cancers, even when HIV is well-controlled with antiretroviral therapy. Despite the tremendous advancements in HIV and cancer care over the past several decades, PWH have lower cancer-related survival compared with the general population. Treating HIV-associated cancers requires a multidisciplinary team to manage concurrent opportunistic infections, potential drug-drug interactions, and the co-occurrence of more than one cancer in the same patient. Many factors may lead PWH to receive inappropriate dose adjustments, exclusion from emerging therapies and clinical trials, or no cancer therapy at all. In general, PWH should receive the same standard, full-dose cancer therapy used in the general population unless there are data for specific cancer regimens in PWH. Agents targeting PD-1 and PD-L1 have US Food and Drug Administration (FDA)-approved indications in many HIV-associated cancers, including Hodgkin lymphoma, cervical cancer, head and neck cancer, hepatocellular carcinoma, and non-small-cell lung cancer; however, PWH were excluded from all clinical trials that led to FDA approval of these agents. Several prospective studies and an international retrospective study of PWH with advanced cancer have shown anti-PD-(L)-1 agents to be safe and effective across expected cancer types and CD4+ T-cell counts, supporting their use in PWH for FDA-approved indications. Learning from the experience in anti-PD-(L)-1 agents, future cancer clinical trials should include and seek to actively enroll PWH, so that they have equal and timely access to emerging cancer therapies.
Conflict of interest statement
The following represents disclosure information provided by the author of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

