Loss of TET2 in human hematopoietic stem cells alters the development and function of neutrophils
- PMID: 37267914
- PMCID: PMC12356769
- DOI: 10.1016/j.stem.2023.05.004
Loss of TET2 in human hematopoietic stem cells alters the development and function of neutrophils
Abstract
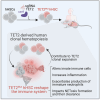
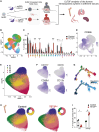
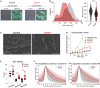
Somatic mutations commonly occur in hematopoietic stem cells (HSCs). Some mutant clones outgrow through clonal hematopoiesis (CH) and produce mutated immune progenies shaping host immunity. Individuals with CH are asymptomatic but have an increased risk of developing leukemia, cardiovascular and pulmonary inflammatory diseases, and severe infections. Using genetic engineering of human HSCs (hHSCs) and transplantation in immunodeficient mice, we describe how a commonly mutated gene in CH, TET2, affects human neutrophil development and function. TET2 loss in hHSCs produce a distinct neutrophil heterogeneity in bone marrow and peripheral tissues by increasing the repopulating capacity of neutrophil progenitors and giving rise to low-granule neutrophils. Human neutrophils that inherited TET2 mutations mount exacerbated inflammatory responses and have more condensed chromatin, which correlates with compact neutrophil extracellular trap (NET) production. We expose here physiological abnormalities that may inform future strategies to detect TET2-CH and prevent NET-mediated pathologies associated with CH.
Keywords: CRISPR; TET2; clonal hematopoiesis; hematopoietic stem and progenitor cells; immune system; preleukemic neutrophil.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Zhu Y.P., Padgett L., Dinh H.Q., Marcovecchio P., Blatchley A., Wu R., Ehinger E., Kim C., Mikulski Z., Seumois G., et al. Identification of an early unipotent neutrophil progenitor with pro-tumoral activity in mouse and human bone marrow. Cell Rep. 2018;24 doi: 10.1016/j.celrep.2018.07.097. 2329.e8–2341.e8. - DOI - PMC - PubMed
-
- Evrard M., Kwok I.W.H., Chong S.Z., Teng K.W.W., Becht E., Chen J., Sieow J.L., Penny H.L., Ching G.C., Devi S., et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018;48 doi: 10.1016/j.immuni.2018.02.002. 364.e8–379.e8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

