5-Azacytidine- and retinoic-acid-induced reprogramming of DCCs into dormancy suppresses metastasis via restored TGF-β-SMAD4 signaling
- PMID: 37267946
- PMCID: PMC10592471
- DOI: 10.1016/j.celrep.2023.112560
5-Azacytidine- and retinoic-acid-induced reprogramming of DCCs into dormancy suppresses metastasis via restored TGF-β-SMAD4 signaling
Abstract
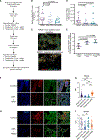
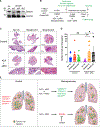
Disseminated cancer cells (DCCs) in secondary organs can remain dormant for years to decades before reactivating into overt metastasis. Microenvironmental signals leading to cancer cell chromatin remodeling and transcriptional reprogramming appear to control onset and escape from dormancy. Here, we reveal that the therapeutic combination of the DNA methylation inhibitor 5-azacytidine (AZA) and the retinoic acid receptor ligands all-trans retinoic acid (atRA) or AM80, an RARα-specific agonist, promotes stable dormancy in cancer cells. Treatment of head and neck squamous cell carcinoma (HNSCC) or breast cancer cells with AZA+atRA induces a SMAD2/3/4-dependent transcriptional program that restores transforming growth factor β (TGF-β)-signaling and anti-proliferative function. Significantly, either combination, AZA+atRA or AZA+AM80, strongly suppresses HNSCC lung metastasis formation by inducing and maintaining solitary DCCs in a SMAD4+/NR2F1+ non-proliferative state. Notably, SMAD4 knockdown is sufficient to drive resistance to AZA+atRA-induced dormancy. We conclude that therapeutic doses of AZA and RAR agonists may induce and/or maintain dormancy and significantly limit metastasis development.
Keywords: CP: Cancer; CP: Cell biology; disseminated cancer cell; dormancy; enhancers; metastasis; transcriptional reprogramming.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.A.A.-G. is a scientific co-founder of, scientific advisory board member at, and equity owner in HiberCell and receives financial compensation as a consultant for HiberCell, a Mount Sinai spin-off company focused on therapeutics that prevent or delay cancer recurrence.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

