Airway T cells are a correlate of i.v. Bacille Calmette-Guerin-mediated protection against tuberculosis in rhesus macaques
- PMID: 37267955
- PMCID: PMC10355173
- DOI: 10.1016/j.chom.2023.05.006
Airway T cells are a correlate of i.v. Bacille Calmette-Guerin-mediated protection against tuberculosis in rhesus macaques
Abstract
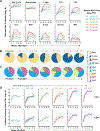
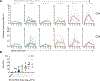
Bacille Calmette-Guerin (BCG), the only approved Mycobacterium tuberculosis (Mtb) vaccine, provides limited durable protection when administered intradermally. However, recent work revealed that intravenous (i.v.) BCG administration yielded greater protection in macaques. Here, we perform a dose-ranging study of i.v. BCG vaccination in macaques to generate a range of immune responses and define correlates of protection. Seventeen of 34 macaques had no detectable infection after Mtb challenge. Multivariate analysis incorporating longitudinal cellular and humoral immune parameters uncovered an extensive and highly coordinated immune response from the bronchoalveolar lavage (BAL). A minimal signature predicting protection contained four BAL immune features, of which three remained significant after dose correction: frequency of CD4 T cells producing TNF with interferon γ (IFNγ), frequency of those producing TNF with IL-17, and the number of NK cells. Blood immune features were less predictive of protection. We conclude that CD4 T cell immunity and NK cells in the airway correlate with protection following i.v. BCG.
Keywords: NK; T cell; adaptive immunity; correlates; lung; mucosal; tuberculosis vaccine.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Martinez L, Cords O, Liu Q, Acuna-Villaorduna C, Bonnet M, Fox GJ, Carvalho ACC, Chan PC, Croda J, Hill PC, et al. (2022). Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: a systematic review and individual participant data meta-analysis. Lancet Glob Health 10, e1307–e1316. 10.1016/S2214-109X(22)00283-2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

