Exclusion of Horizontal and Vertical Transmission as Major Sources of Trypanosoma Cruzi Infections in a Breeding Colony of Rhesus Macaques (Macaca Mulatta)
- PMID: 37268411
- PMCID: PMC10290485
- DOI: 10.30802/AALAS-CM-23-000005
Exclusion of Horizontal and Vertical Transmission as Major Sources of Trypanosoma Cruzi Infections in a Breeding Colony of Rhesus Macaques (Macaca Mulatta)
Abstract
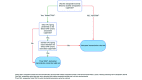
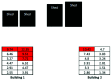
The vector-borne protozoal parasite Trypanosoma cruzi causes Chagas disease in humans and animals. This parasite is endemic to the southern United States where outdoor-housed NHP at biomedical facilities are at risk of infection. In addi- tion to the direct morbidity caused by T. cruzi, infected animals are of limited biomedical research use because infections can produce confounding pathophysiologic changes even in animals with no clinical disease. In part due to concerns for direct T. cruzi transmission between animals, infected NHP at some institutions have been culled, removed, or otherwise isolated from uninfected animal populations. However, data that document horizontal or vertical transmission in captive NHP in the United States are not available. To evaluate the potential for inter-animal transmission and to identify environmental factors that affect the distribution of new infections in NHPs, we conducted a retrospective epidemiologic study of a rhesus macaque ( Macaca mulatta ) breeding colony in south Texas. We used archived biologic samples and husbandry records to identify the time and location of macaque seroconversion. These data were used to perform a spatial analysis of how geographic location and animal associations affected the spread of disease and to infer the importance of horizontal or vertical routes of transmission. The majority of T. cruzi infections were spatially clustered, suggesting that environmental factors promoted vector exposure in various areas of the facility. Although we cannot not rule out horizontal transmission, our data suggest that horizontal transmission was not a critical route for spread for the disease. Vertical transmission was not a contributing factor in this colony. In conclusion, our findings suggest that local triatome vectors were the major source of T. cruzi infections in captive macaques in our colony. Therefore, limiting contact with vectors, rather than segregation of infected macaques, is a key strategy for disease prevention at institutions that house macaques outdoors in the southern United States.
Figures



Similar articles
-
Reproductive Outcomes in Rhesus Macaques (Macaca mulatta) with Naturally-acquired Trypanosoma cruzi Infection.Comp Med. 2020 Apr 1;70(2):152-159. doi: 10.30802/AALAS-CM-19-000077. Epub 2020 Mar 17. Comp Med. 2020. PMID: 32183928 Free PMC article.
-
Trypanosoma cruzi Transmission Among Captive Nonhuman Primates, Wildlife, and Vectors.Ecohealth. 2018 Jun;15(2):426-436. doi: 10.1007/s10393-018-1318-5. Epub 2018 Mar 1. Ecohealth. 2018. PMID: 29497880 Free PMC article.
-
Chagas disease in 2 geriatric rhesus macaques (Macaca mulatta) housed in the Pacific Northwest.Comp Med. 2014 Aug;64(4):323-8. Comp Med. 2014. PMID: 25296019 Free PMC article.
-
Trypanosoma cruzi and Chagas' Disease in the United States.Clin Microbiol Rev. 2011 Oct;24(4):655-81. doi: 10.1128/CMR.00005-11. Clin Microbiol Rev. 2011. PMID: 21976603 Free PMC article. Review.
-
Chagas Disease Ecology in the United States: Recent Advances in Understanding Trypanosoma cruzi Transmission Among Triatomines, Wildlife, and Domestic Animals and a Quantitative Synthesis of Vector-Host Interactions.Annu Rev Anim Biosci. 2022 Feb 15;10:325-348. doi: 10.1146/annurev-animal-013120-043949. Epub 2021 Nov 10. Annu Rev Anim Biosci. 2022. PMID: 34758274 Review.
Cited by
-
Two-dimensional QSAR-driven virtual screening for potential therapeutics against Trypanosoma cruzi.Front Chem. 2025 Jun 11;13:1600945. doi: 10.3389/fchem.2025.1600945. eCollection 2025. Front Chem. 2025. PMID: 40568639 Free PMC article.
-
Chagas Disease, an Endemic Disease in the United States.Emerg Infect Dis. 2025 Sep;31(9):1691-1697. doi: 10.3201/eid3109.241700. Emerg Infect Dis. 2025. PMID: 40866797 Free PMC article. Review.
-
Development of an operational trap for collection, killing, and preservation of triatomines (Hemiptera: Reduviidae): the kissing bug kill trap.J Med Entomol. 2024 Nov 14;61(6):1322-1332. doi: 10.1093/jme/tjae087. J Med Entomol. 2024. PMID: 39024462 Free PMC article.
References
-
- Anselin L, Syabri I, Kho Y. 2006. GeoDa: An introduction to spatial data analysis. Geogr Anal 38:5–22. 10.1111/j.0016-7363.2005.00671.x. - DOI
-
- Araujo PF, Almeida AB, Pimentel CF, Silva AR, Sousa A, Valente SA, Valente VC, Britto MM, Rosa AC, Alves RM, Hagström L, Teixeira AR. 2017. Sexual transmission of American trypanosomiasis in humans: A new potential pandemic route for Chagas parasites. Mem Inst Oswaldo Cruz 112:437–446. 10.1590/0074-02760160538. - DOI - PMC - PubMed
-
- Beatty NL, Forsyth CJ, Burkett-Cadena N, Wisely SM. 2022. Our current understanding of chagas disease and Trypanosoma cruzi infection in the state of Florida — An update on research in this region of the USA. Curr Trop Med Rep 9:150–159. 10.1007/s40475-022-00261-w. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

