Restoration of Sleep and Circadian Behavior by Autophagy Modulation in Huntington's Disease
- PMID: 37268416
- PMCID: PMC10312063
- DOI: 10.1523/JNEUROSCI.1894-22.2023
Restoration of Sleep and Circadian Behavior by Autophagy Modulation in Huntington's Disease
Abstract
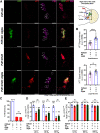
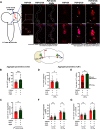
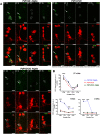
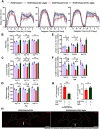
Circadian and sleep defects are well documented in Huntington's disease (HD). Modulation of the autophagy pathway has been shown to mitigate toxic effects of mutant Huntingtin (HTT) protein. However, it is not clear whether autophagy induction can also rescue circadian and sleep defects. Using a genetic approach, we expressed human mutant HTT protein in a subset of Drosophila circadian neurons and sleep center neurons. In this context, we examined the contribution of autophagy in mitigating toxicity caused by mutant HTT protein. We found that targeted overexpression of an autophagy gene, Atg8a in male flies, induces autophagy pathway and partially rescues several HTT-induced behavioral defects, including sleep fragmentation, a key hallmark of many neurodegenerative disorders. Using cellular markers and genetic approaches, we demonstrate that indeed the autophagy pathway is involved in behavioral rescue. Surprisingly, despite behavioral rescue and evidence for the involvement of the autophagy pathway, the large visible aggregates of mutant HTT protein were not eliminated. We show that the rescue in behavior is associated with increased mutant protein aggregation and possibly enhanced output from the targeted neurons, resulting in the strengthening of downstream circuits. Overall, our study suggests that, in the presence of mutant HTT protein, Atg8a induces autophagy and improves the functioning of circadian and sleep circuits.SIGNIFICANCE STATEMENT Defects in sleep and circadian rhythms are well documented in Huntington's disease. Recent literature suggests that circadian and sleep disturbances can exacerbate neurodegenerative phenotypes. Hence, identifying potential modifiers that can improve the functioning of these circuits could greatly improve disease management. We used a genetic approach to enhance cellular proteostasis and found that overexpression of a crucial autophagy gene, Atg8a, induces the autophagy pathway in the Drosophila circadian and sleep neurons and rescues sleep and activity rhythm. We demonstrate that the Atg8a improves synaptic function of these circuits by possibly enhancing the aggregation of the mutant protein in neurons. Further, our results suggest that differences in basal levels of protein homeostatic pathways is a factor that determines selective susceptibility of neurons.
Keywords: Atg8a; Drosophila circadian circuit; Huntington's disease; autophagy; pigment dispersing factor; sleep.
Copyright © 2023 the authors.
Figures





References
-
- Allocca M, Zola S, Bellosta P (2018) The fruit fly, Drosophila melanogaster: modeling of human diseases (Part II). In: Drosophila melanogaster: model for recent advances in genetics and therapeutics. London, United Kingdom: IntechOpen.
-
- Bellosta Diago E, Pérez Pérez J, Santos Lasaosa S, Viloria Alebesque A, Martínez Horta S, Kulisevsky J, López del Val J (2017) Circadian rhythm and autonomic dysfunction in presymptomatic and early Huntington's disease. Parkinsonism Relat Disord 44:95–100. 10.1016/j.parkreldis.2017.09.013 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
